










 Official publication of the Czech Society of Ultrasound in Obstetrics and Gynecology.
Official publication of the Czech Society of Ultrasound in Obstetrics and Gynecology.

Chronic pelvic pain syndrome (CPPS) is a fairly common disorder in women (1-3). Vulvodynia is the most common one (4,5). The International Pelvic Pain Society (IPPS) adopted a new vulvar pain and vulvodynia terminology and classification based on evidence-based information about the disease (6,7). It enumerates the types of pelvic pain linked to many organic diseases and accepts the condition of idiopathic vulvodynia without any organic reason (4,8).
Idiopathic vulvodynia is a chronic pain syndrome affecting the vulvar area. Symptoms typically include chronic pain, burning, rawness, soreness, or irritation in the vaginal area, with no identifiable cause (9). Myofascial pain, pelvic hypertonia and trigger points are other conditions of remarkable interest. Standard therapy consists in treating neuropathic pain. Other treatments include pelvic floor rehabilitation combined with surface electromyography, interferon alpha, estrogen creams and botulinum toxin A (10).
If conservative treatment is ineffective, surgery can be recommended, mainly surgical denervation of the vulva. The blockade of trigger points with a local anesthetic is also performed. Most authors advocate a therapy-based approach. Many patients are routinely treated as if they had an infection, although no infection has been identified or confirmed. Only rarely complete relief from subjective patient complaints can be achieved (10). The fundamental problem in patients is the long-lasting pain that severely affects their quality of life (11,12). Muscle and fascial pain are commonly diagnosed in general practice. Recent studies were based on functional principles such as an expression of proteins and muscle metabolism that extend the role of peripheral factors in maintaining chronic pain (13,14). Published studies pointed to a higher concentration of end products and violating role of local hypoxia on oxidative metabolism. An insufficient capacity for muscle recovery is often presented. An inflammation and metabolic pathway changes are blamed for it (15). Long-standing hypoxia in perineal muscles could cause muscular and perimuscular impairments, leading to long-term pain not sufficiently reacting to common therapy. We registered hypoxia in acute pelvic pain in our former study on dyspareunia (16). In our previous study we treated vulvodynia by focused shock wave Storz device (13,17). We accidentally identified significantly low pulse oximetry levels in perigenital region of affected women. We decided to focus on this area to explain the hitherto unknown reason for the change. We compared pulse oximetry oxygen saturation (SpO2) of vulvodynia affected women (treatment group) to the group of pain absent healthy individuals (controls).
A retrospective study design was adopted. Study data were collected from women who participated in our previous research on vulvodynia, conducted between 2015 and 2018 (18). All study procedures adhered to the principles of the 1964 Helsinki Declaration. The study protocol was approved by the Ethical Committee of the Teaching Hospital at Charles University, Prague. All patients provided written informed consent for participation in the source study.
The treatment group comprised 31 women diagnosed with vulvodynia, aged 24–52 years (mean age 40; SD = 8.3). Objective vulvodynia was defined as persistent vulvar pain lasting for at least three months within the previous six months. The control group included 31 women, aged 25–53 years (mean age 39; SD = 9.6), with no history of relevant medical conditions. They were recruited from volunteers undergoing routine preventive examinations. Both groups were similar in baseline demographic characteristics, potential confounders, parity, and BMI, as mentioned in the source study. The appropriate sample size was determined using IBM Sample Power.
SpO₂ levels were measured during routine gynecological examinations in both groups. Measurements were taken in the vulvo-perineal posterior area and the bulbospongiosus muscle. SpO₂ was determined by assessing the absorption differences between two wavelengths: a red beam at 660 nm and near-infrared (IR) light at 940 nm. Light passing through the tissue was detected on the opposite side. Pulse oximetry determined oxyhemoglobin saturation by analyzing the differential absorption of infrared (IR) and red light (17,19,20). SpO₂ detection was specific to arterial blood, relying on fluctuations in absorbed IR and red light during the cardiac cycle. The blood volume in veins, capillaries, skin, fat, and bones remained relatively constant (17). A standard anesthesiology device was used for the measurement.
Data normality was assessed using kurtosis, skewness, and the Shapiro-Wilk test. Depending on the normality results, either parametric or nonparametric tests were applied to compare SpO₂ means and medians between the two groups. Statistical analyses were performed using IBM SPSS, and IBM Sample Analysis was used to retrospectively confirm sample size adequacy.
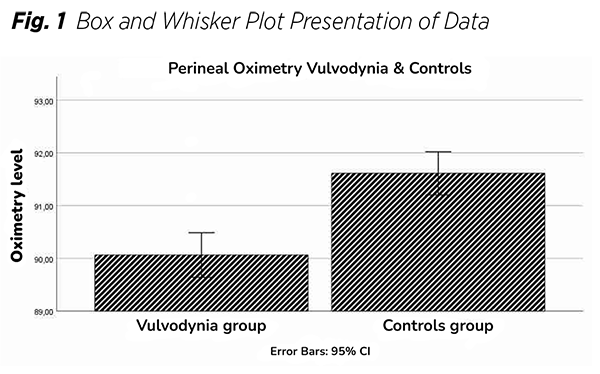
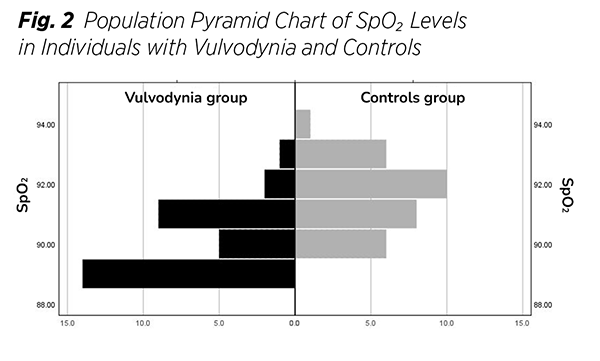
The study included 31 participants per group. The main characteristics are presented in (Tab. 1). Charts illustrating the distribution of SpO₂ values for both groups are also provided (Fig. 1), (Fig. 2). Data normality was evaluated using skewness and kurtosis statistics, along with the Shapiro-Wilk test. Since the data did not follow a Gaussian distribution, the nonparametric Mann-Whitney U test was employed for comparisons.
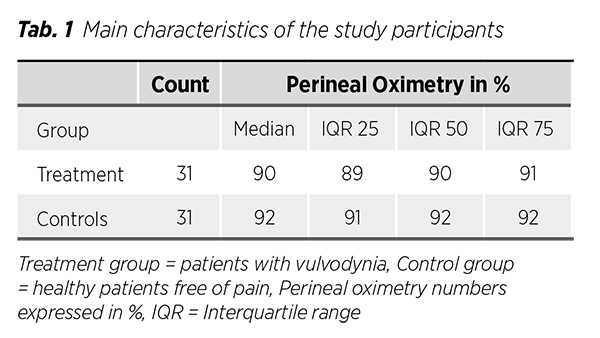
The primary nonparametric values reported were medians and Interquartile-ranges (IQR), A perineal oximetry comparison between the placebo and treatment groups was conducted using the Mann-Whitney U test. Their difference was statistically significant (p < 0.001). The median SpO₂ was 90% in the treatment group and 92% in the control group. Variability was comparable between both groups, Cohen’s d was greater than 0.8, indicating a large effect size.
Sample size calculations confirmed that a two-sided test with α = 0.05 had at least 80% power to detect a true effect. Based on our data, a minimum of 20 participants (10 per group) was required, which was considerably lower than the actual study sample size (n = 62).
In our study we found decreased oxygenation in painful perigenital areas of women. Lower tissue oxygenation is thought to be present in tender point spots due to local ischemia. Some studies confirmed the change in local microcirculation of affected women. Altered microcirculation may contribute to muscle pain in vulvodynia (13). It is reported that regulation of the microcirculation in fibromyalgia is disturbed in a manner that could result in sensitization of the intramuscular nociceptors (15). A reduced capillary density in tender areas has been documented. Histological findings could detect “moth-eaten” and/or “ragged” red fibers that could indicate the abnormal growth of mitochondria. Their pathological distribution could be caused by local hypoxia, mainly caused by hypoxemia, lowered blood flow supply in affected spots (14,15). A plausible reason of hypoxia may be low physical inactivity resulting in mitochondrial insufficiency with abnormal metabolites levels. Deconditioning is the term often used in this context. Pain-reduced daily activity could cause deconditioning, which could complete the image of biochemical and anatomical changes in the muscle (13,21). As far as we know, this is the first study focused on oximetry regarding the context of vulvodynia.
The advantage of our study is a straightforward, painless, low-cost and time-saving technique.
It could be repeated without significant problems. The main disadvantage is the lack of standards and guidelines for implementing this procedure.
Idiopathic vulvodynia is a chronic pain condition that remains poorly understood and often resistant to conventional therapies. In the course of the examination, we observed clinically meaningful decreased levels of SpO₂. Pelvic oximetry was compared between vulvodyniaaffected women (treatment group) and the control group. This comparison proved significant important hypoxia in the perigenital muscle area of affected women (p < 0.001). The hypoxia in chronic pelvic pain vulvodynia seems to be present the same way as hypoxia in acute pelvic pain dyspareunia in our previous study. These results can help understand the trigger of this kind of pain and target our treatment power. We can consider oxygen therapy, vasodilators, or possible targeted rehabilitation.
Ethical approval: Teaching hospital of Charles University Prague 3.8.2021/10150/EK-Z
Registration number of the source study: 3.8.2015/7770/ EK-Z. All procedures performed in the study were in accordance with ethical standards and in an agreement with the 1964 Helsinki Declaration and its later amendments.
Conflict of interest: The authors declare that they have no conflict of interest.
Funding sources: The study did not receive any funding. The authors have nothing to disclose.
Acknowledgements: We would like to express thanks to our colleagues and staff without whose help this work would not be created and who put the extra into ordinary.