










 Official publication of the Czech Society of Ultrasound in Obstetrics and Gynecology.
Official publication of the Czech Society of Ultrasound in Obstetrics and Gynecology.

The human body can experience pain or muscle spasms due to a wide range of factors. The pursuit of optimal pain management and alleviation has led to the use of botulinum toxin (BTX).
Aim: To describe the characteristics and pathophysiology of BTX. The efficacy of BTX was assessed through a meta-analysis of clinical trials employing placebo controls. Due to the unavailability of controlled trials on the vulvo-perineal area, we opted to replace them with controlled trials conducted on striated muscles in various human body regions. We assume that the biochemical reaction is either identical or very similar. This paper outlines our proposed recommendations regarding applications in the vulvo-perineal region, including the corresponding dosage.
Methods and Patients: A review of clinical trials pertaining to BTX was performed. Databases from 2000 to 2023 were evaluated. No placebo-controlled studies were conducted specifically on the muscles of the vulvo-perineal area. Nine trials of patients with muscle spasticity after stroke fulfilled the criteria. Patients were treated with BTX A Botox or Dysport. The assessment of muscle spasticity change involved calculating the mean difference of the Modified Ashworth Scale (MAS) before and 4-6 weeks post-treatment. Cohen's d was employed to indicate the effect size. The overall study's effect size was 0.97; P<.001, indicating a large effect size of the meta-analysis. Homogeneity was not distorted, P = 0.076. Heterogeneity implies slight distortion, I2 = 46%. The forest plot confirms this finding. The Funnel Plot encompasses a wide range of studies, suggesting consistent findings and limited bias.
The dosage of BTX pertains to the fact that commonly used Botox and Dysport have a calculation ratio of 1 IU to 2.5 IU. A Botox dosage ≤400 units is considered safe, with an odds ratio (OR) of 0.97 without a notable rise in adverse events (AEs); a dosage of >600 units has an OR of 2.98, accompanied by a notable increase in AEs. The Botox dosage for the vulvo-perineal area is approximately 100 IU divided into several punctures. After one month, a booster dose is available.
Discussion: We summarized the fundamental physiopathology of BTX and demonstrated its efficacy in mitigating pain and spastic pain among patients. We presented a suitable dosage of BTX in the vulvo-perineal area and discussed potential dosage limitations.
Pelvic pain disorder is a condition that often occurs in women. Chronic pelvic pain syndrome (CPPS) has many subdivisions which relate to the source of origin. CPPS is estimated to affect 10% of the population. Different traumas, contusions, strains, and sprains could induce pain in striated skeletal muscles. We do not name all the diseases of this area whose main symptom is pain or spastic pain (1-3). Higher concentrations of histamine, serotonin, bradykinin, and substance P, in combination with local ischemia and swelling, give rise to pain and local sympathetic symptoms (4). Referred pain with origin in striated muscles could also affect smooth muscle organs. This pain is often based on the principle of Head‘s zones. One common speculation suggests that the neuromuscular junction exhibits an abnormal increase in acetylcholine secretion (5).
The absence of optimal solutions and a propensity to seek a method that not only mitigates discomfort during medical consultations resulted in the integration of botulinum toxin as a reliable pain alleviation method (6-8).
Our contribution includes a survey and pathophysiology of botulinum toxin (BTX). We aim to present the research part with a meta-analysis of placebo-controlled studies to evaluate the efficacy of this drug on a larger group of patients treated in clinical studies. We intend to primarily focus on conducting placebo-controlled trials involving vulvo-perineal muscles. If controlled trials of this area were unavailable because of a lack or absence of placebo studies on this intimate area, we would anticipate a similar reaction of striated muscles located in a different body area. We suppose that the response of striated muscles on BTX should be the same or similar because of their identical physiology and structure. Moreover, BTX has successfully treated striated muscles in various body parts, including the face and the lower extremities. Here, we always refer to intramuscular or tendon-muscular injection applications. Therefore, we believe a meta-analysis of this nature should serve the same intention. Additionally, we would like to present a recommended application dosage for the vulvoperineal area that is still functional but free of adverse events.
BTX is a neurotoxic protein produced by Clostridium botulinum, the Gram-positive anaerobic spore-forming organism, preventing the release of acetylcholine (ACh) from an axon at the neuromuscular junction (8-10). The name originates from the Latin term „botulus“, meaning „sausage“. The illness was first identified in Germany among individuals who had consumed contaminated sausage and was initially referred to as „sausage poisoning“. Seven immunological subtypes from A to G are present, with A and B being used in the context of human biology. BTX blocks the release of ACh and other transmitters from presynaptic endings. This process leads to lesser contractility and muscle relaxation. The BTX does not cross the bloodbrain barrier, and the side effects are slight if administered locally, not intravenously. BTX acts solely on motor nerve endings, leaving sensory nerve endings untouched. In some studies, the analgesic effect of BTX is highlighted, but from attributed influences such as improved blood flow, the release of compression and improved oxygenation of the affected area (6,11,12).
Botulinum toxin is commercially synthesized from bacterial cytosol as a single-chain peptide with a molecular weight of approximately 150 kilo Daltons (Kd). Further cleavage separates this chain into two smaller ones, 100 and 50 Kd, which remain linked and potent for pharmaceutical use in the desired treatment.
There are many indications for the clinical use of this agens. It manifests in various conditions, such as blepharospasm, strabismus, cervical dystonia, bladder disorder, chronic anal fissure treatment and pelvic pain. Vulvodynia and vaginismus are frequently cited among them. Spastic statuses, including neurological disorders, are significant areas of its use (8,13,14). Regarding cosmetic indications, we would like to highlight the treatment of wrinkles, resurfacing, and popular hyperhidrosis treatment. Cosmetic treatment has recently been considered the most frequent BTX application (15). Several other applications are still being considered.
Botulinum toxin A (BTXA) is the most employed treatment for spasms. The typical representatives of BTXA are Dysport and Botox. Other drugs produced are also cited.
We sought to prove the efficacy of BTX in clinical studies with placebo-checked controls (10).
A comprehensive review of clinical trials pertaining to BTX was performed. Numerous studies were conducted, yet only a few met the criteria of placebo-controlled trials. We checked databases from 2000 to 2023. We included all studies that used BTXA. The chosen drugs were Botox and Dysport. Other medications, such as BTXB, were not included due to a lack of compliance. But, there were only few of them. None of placebo controlled BTX suitable studies dealt with the vulvo-perineal area.
It was found that the only relevant studies were those involving individuals with spasticity after experiencing a stroke. Consequently, we decided to perform our metaanalysis using data acquired from studies on spastic patients to generalize the results on a wide range of striated muscles, including the vulvo-perineal area.
Our research specifically examined individuals who participated in placebo-controlled trials.
They experienced moderate to severe spasticity in their lower or upper extremities within the past three months following a cerebrovascular event. The severity was defined with the MAS (Modified Ashworth Scale, 0-5 points), designed for muscle tone evaluation in the upper and lower extremities. The patients were randomly assigned to either the treatment or placebo group according to their placebocontrolled study protocols. Regrettably, a more detailed depiction of the participants cannot be provided. It would influence the coherence of the article and prolong the reading duration. The paper references these studies, which were reviewed and published in their respective databases.
Patients were treated with intramuscular injections of Dysport 500-1500 units or Botox 200-300 units. The dosage effect was practically identical. Nevertheless, the differentiation necessitates an explanation in the following section of this document. It typically adheres to the suggested calculation ratio of 2.5:1. Change in muscle spasticity was checked 4-6 weeks after treatment. The difference in mean values of MAS between the onset and follow-up period was used to quantify the change in improvement for both the treatment and control groups. It simply denoted the number expressing improvement (or no improvement) in the patient’s status. Standard deviation (SD), size and other pertinent information were also provided. According to the protocol of the included studies, other continual anti-spastic treatment was not allowed nor administered. The heterogeneity of the studies is exclusively determined within our meta-analysis, considering their impact on the overall outcome and their proportion in relation to the total 100% result.
A meta-analysis was conducted using CMA (comprehensive meta-analysis) and SPSS software. Results are provided with Cohen’s d for individual studies and the overall effect size for the entire analysis. Tests for homogeneity and heterogeneity were provided. The forest plot visually illustrates the impact of individual studies on the overall outcome, while the funnel plot offers an overview of the included studies and their heterogeneity.
The research results were calculated from nine studies that met the abovementioned inclusion criteria. Studies that lacked reporting of means and standard deviations or variance or where these values could not be derived from the provided data were not included.
The meta-analysis includes the following nine studies: Bakheit et al. 2000, 2001; Smith et al. 2000; Brashear et al. 2002; Childers et al. 2004; Gracies et al. 2015, two studies; Esquenazi et al. 2020, two studies (8,16-21). Two studies (Esquenazi et al. 2020) were limited to the lower extremities, while the remaining were limited to the upper extremities. The dosage of BTXA varied between 200 and 300 units in Botox and 500 to 1500 units in Dysport.
Results of our Meta-analysis study are presented in the following table (Tab. 1).
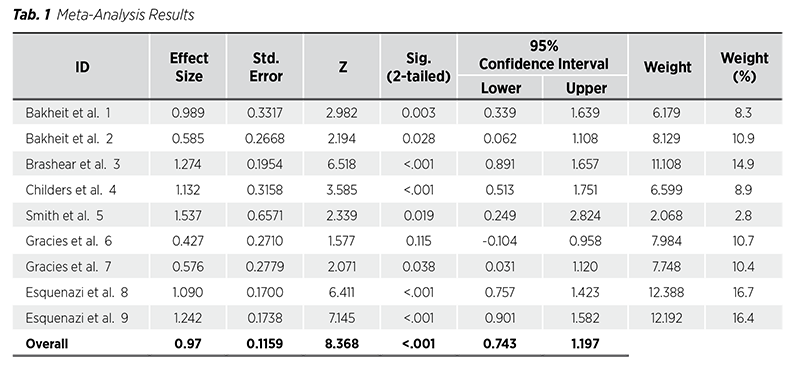
The first function column in the results table presents Cohen’s d values for the participating studies and the overall effect for the entire study. In the second column, standard errors are presented. Z value is mentioned in the third column. The fourth column is devoted to p-values. The lower and upper confidence intervals (CIs) are presented in the fifth and sixth columns, respectively, with the same values for the entire study presented at the bottom. The seventh column presents individual study weight, and the last, eighth column expresses the percentage of the influence on the overall study results in percents from 100%. The overall effect size of the study is 0.97 with p<0.001. According to Cohen, a small effect size is denoted by 0.2, a medium effect size by 0.5, a large effect size by 0.8, and a very large effect size by 1.3. We would like to highlight that study 6 is the only one lacking a significant effect, and where CI is minus, rendering it insignificant.
The study‘s homogeneity remains intact. A p-value of 0.076 indicates a lack of statistical significance, thereby suggesting the absence of homogeneity distortion.
The measure of heterogeneity describes how cohesive particular complements of the study are.
For the main interpretation, I2 is used.
A rough interpretation is as follows:
0%-30% = not important
>30%-60% = moderate heterogeneity
>60%-80% = substantial heterogeneity
>80%-100% = considerable heterogeneity
Our I2 = 46%, i.e., moderate heterogeneity. The distortion of the study is mild, acceptable for a correct interpretation. There is no need to expel any study part.
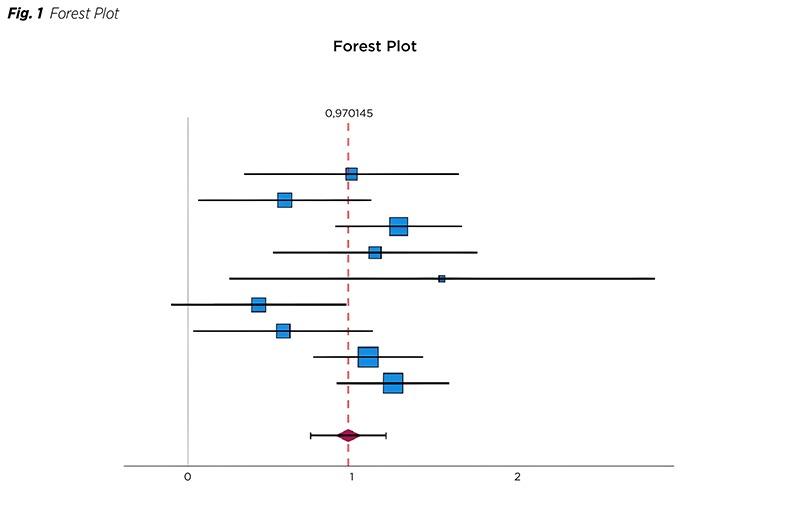
The Forest plot describes the graphic interpretation of the participating studies. The boxes represent the study sizes, while the CI whiskers indicate the extent of the study‘s dispersion. The red structure known as a ‚diamond‘ in the lower section represents the overall impact of the study, as indicated by its CI. Study 6‘s weakness is evident even in this context, as it exceeds the fundamental zero point (Fig. 1).
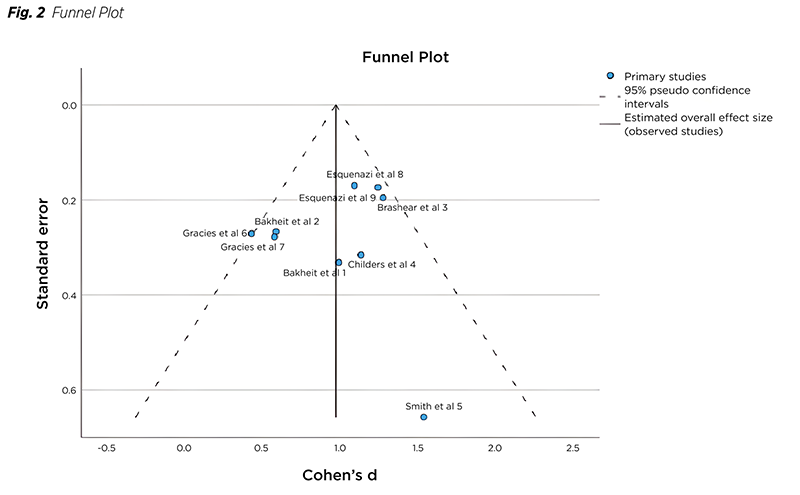
The Funnel plot visually represents the distribution of studies, with Cohen‘s d on the X axis and the standard error on the Y axis (Fig. 2). Except for study 6, which could be regarded as an outlier and the most diverse element of the study, all other studies fall within the purview of the funnel plot.
We would achieve significantly improved outcomes by hypothetically excluding study 6 based on the reasons above. Cohen‘s d‘s value of 1.048, the absence of distorted heterogeneity with I2 being 25.1% would be present. The funnel plot would completely cover all the studies. Not excluding the study 6 is a tax on completeness.
Botulinum toxin dosage and recommendations and occurrence of adverse events:
OnabotulinumtoxinA (Botox®)
AbobotulinumtoxinA (Dysport®)
IncobotulinumtoxinA (Xeomin®)
BotulotoxinA is probably the most frequently used BTX drug for human applications. Some clinicians use group B, but setting the best drug concentrations is challenging. The most commonly used products are onabotulinumtoxinA (Botox®), abobotulinumtoxinA (Dysport®), and incobotulinumtoxinA (Xeomin®), with no particular order specified in the citation.
It is crucial that, unlike common dosage similarities of other drugs, they differ greatly. The concentrations are different in all the BTXs. When we compare onabotulinumtoxinA (Botox®) with abobotulinumtoxinA (Dysport®), it is mostly described as a calculation ratio of 1:2.5 International Units (IU). IncobotulinumtoxinA (Xeomin®) is remarkably similar to Botox.
Formerly, the concentration of BTX was quantified in MU (mouse units), which denotes the lethal dose needed to kill one mouse. The terminology on IU was altered for political correctness and denoted the minimal molecular quantity required to trigger a clinical response in humans. In all the studies mentioned above, our focus is solely on IU. To avoid any possible confusion, we have decided to refrain from providing medication recommendations and, instead, direct our exclusive attention towards the concentration of Botox. If necessary, the reader may calculate them using the formula mentioned. The concentrations are divided into three groups: ≤400 units, 401-600, and >600 units, which refer to lower, moderate, and high concentrations, respectively. Adverse events described after the highest administrations are dysphagia, weakness, discomfort, virus-like syndrome, transient local pain, or fatigue. The manifestation of these symptoms is observed at dosages exceeding 600 IU. Adverse events-evaluation studies depict an odds ratio (OR) of 0.97 (95% confidence interval (CI) 0.31, 2.98) for concentrations ≤400 units and an OR 2.98 (95% CI 1.12, 8.13) for concentrations >600 units. Only the second OR for higher concentrations is statistically significant (11,22,23). Lower doses are without substantial adverse events. Vaginal disorders, including vulvodynia, dyspareunia, vaginism, and other vagino-perineal disorders, are mentioned as potential targets of BTX treatment. Dysmenorrhea and pelvis hypertonicity are also referenced (24).
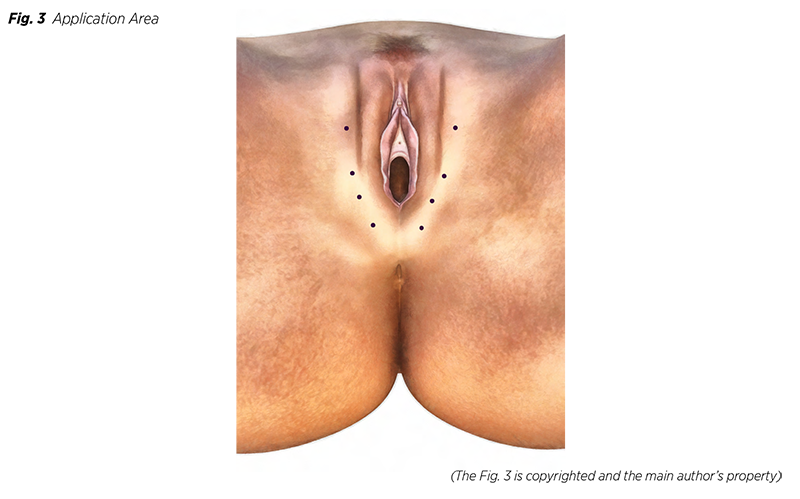
One session‘s dose in this application is much less than those used to treat larger body areas. Areas recommended for the application are puborectal and pubococcygeal muscles or those muscles involved in pain. If deemed necessary, the procedure can be conducted with the use of conscious sedation. When considering Botox BTX, 100 IU per the producer‘s recommended dilution is advised (25). Besides, Botox is handed in 100 IU original bottles. The total amount is divided into roughly eight fractions administrated into intended areas bilaterally (Fig 3). If required, the possibility of a booster application of BTX after a month should be considered while ensuring that the additional dosage does not exceed 100 IU again. To spare the reader, we omit the mention of all the customary recommendations typical for these procedures.
This review aimed to evaluate the potential effects of botulinum toxin on spasticity and pain relief in women with spastic disorders when applied to striated perineal muscles. We frequently receive inquiries regarding the effectiveness of BTX, with concerns about whether the enhanced outcome is simply a placebo effect. Despite engaging in debates and referencing numerous studies, we always hear the same question: ‘This was not proven in the clinical trial with the placebo control group’. Hence, we sought to substantiate our viewpoint in this manner. BTX is commonly employed to treat striated muscles within the human body. We searched for complied clinical trials with placebo-controlled groups that presented their results in a comparable form. Limited research has been conducted on patients with spasticity that compares patients treated with BTX to those given a placebo. Regrettably, there is a lack of research on applying BTX in the pelvic region. If BTX applies to striated muscles, it is plausible to consider its potential application to other muscular areas of a similar histological nature. We can find the similarity with the effect of local anesthetics on the different parts of the body. The only suitable studies on patients with spasticity that employed placebo control were conducted using this method. A thorough investigation was carried out to identify pertinent studies according to the conditions above, resulting in the inclusion of only nine studies that fulfilled the predetermined criteria.
Our meta-analysis yielded statistically significant findings, demonstrating the efficacy of BTX in treating spasticity and the relief of pain in striated muscles. We expect to observe a comparable effect in the vulvo-perineal region. This particular usage has been previously published, albeit without statistical backing. A few notable points should be mentioned. Publication bias arises when trials that yield statistically significant results are consistently published in renowned databases (26).
Publication and selection bias affect especially small trials involved in meta-analysis.
Small trials are more likely to present larger effect than those of larger size (27).
The main drawback of these studies and treatments is the sizeable difference in BTX concentration doses among different manufacturers. The accuracy of the drug‘s clearing process must be considered to ensure the optimal and safest drug.