










 Official publication of the Czech Society of Ultrasound in Obstetrics and Gynecology.
Official publication of the Czech Society of Ultrasound in Obstetrics and Gynecology.

Background: The main objective of this single-center study was to analyze the perioperative complications following minimally invasive surgery (MIS) for uterine cancer, as well as the overall survival and recurrence rate in low-income countries.
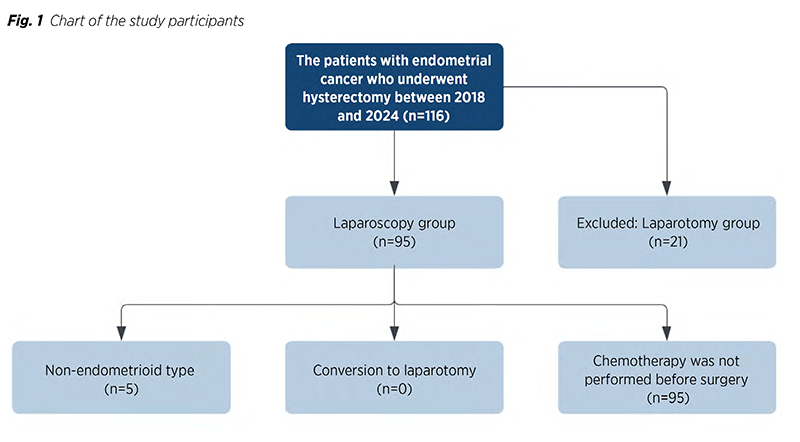
Methods: This retrospective study was conducted with the records of 117 endometrial cancer patients who had surgery between 2018 and 2024 were reviewed. Inclusion criteria encompassed both endometrioid and non-endometrioid cancer types, histological grades I-III, and assessment of pelvic and periaortic lymph nodes. Among the patients, 95 underwent laparoscopic pelvic lymph node dissection and hysterectomy. The study excluded 22 women who had laparotomic hysterectomy with dissection of pelvic and aortic lymph nodes.
Results: Ninety-five patients underwent MIS for endometrial cancer. The most common procedures, performed in ninety-two (96.9%) patients, were TLH, BSO, and BPLND. Sixty-nine (72.6%) patients had a BMI over 30. There were no conversions from laparoscopic surgery (LPS) to laparotomic (LPT). In 65 (68.4%) cases, the procedure took over 120 minutes, and 74 (77.9%) patients had hospital stays of 4 days or less. The longest follow-up was 77 months, with twelve (12.6%) recurrences and an 87.4% (n=83) survival rate.
Conclusion: Our study indicates that LPS is effective for treating uterine-confined endometrial carcinoma, offering comparable lymph node dissection to laparotomy, shorter hospital stays, and fewer postoperative infections for experienced surgeons. While prospective randomized controlled studies are needed to confirm these findings, our results serve as a valuable clinical reference. MIS should be promoted for endometrial cancer treatment, especially in low-income countries.
Open laparotomy has historically been used for full surgical staging of endometrial carcinoma (1). This procedure includes total abdominal hysterectomy (TAH), bilateral salpingo-oophorectomy (BSO), bilateral pelvic and paraaortic lymph node dissection (BPPALND), and peritoneal cytology (2). Traditionally, patients underwent exploratory laparotomy through a midline incision. Surgical techniques for endometrial carcinoma have changed over time (3,4). Furthermore, different institutions and periods have changed how retroperitoneal lymph node evaluation is done. Laparoscopic surgery (LPS), due to its advantages, is becoming a more appealing option to traditional methods for treating gynecologic malignancies particularly in endometrial tumors (5). LPS is gradually taking the place of laparotomy as the standard method of treating endometrial cancer (6). Numerous studies have demonstrated that LPS may be preferred to open surgery since it has decreased risk following surgery, a shorter duration of hospitalization, reduced problems following surgery, less discomfort, and an improved quality of life as a result of a quicker recovery (1). Obesity, hypertension, and diabetes are common co-morbidities among endometrial cancer patients (7). Patients undergoing abdominal surgery are consequently at a higher risk of complications (8,9). Laparoscopic procedures are over the before mentioned drawbacks (5). The laparoscopic method does not alter the incidence of recurrences or the overall survival, despite the fact that long-term risks of recurrence and survival following using uterine manipulator for endometrial cancer have not been detailed (4). By using a uterine manipulator, cranial traction on the uterus is ensured, leading to an easy and more comfortable surgery (4). Due to direct contact with the tumor in the endometrial cavity and the potential for malignant cells to spread, the use of an intrauterine manipulator has been criticized for possibly worsening oncologic outcomes (10,11).
This main objective of this single-center study was to analyze the perioperative complications following minimally invasive surgery (MIS) for endometrial cancer, as well as the overall survival and recurrence rate.
This retrospective investigation was performed at the Department of Oncology at Azerbaijan Medical University conducted. A retrospective review of the records of 117 patients with endometrial cancer who had surgery between 2018 and 2024 was conducted. The research‘s inclusion criteria included cases with both endometrioid and non-endometrioid histological types of cancer, patients with histological grades I-III, and patients having their pelvic and periaortic lymph nodes assessed. A total of 117 patients had LPS and laparotomic (LPT) procedures performed. Preoperatively, the doctor or the patient made the decision between LPS and LPT approaches based on the patient‘s parameters and preferences. There were 95 endometrial cancer patients who had pelvic lymph node dissection and LPS hysterectomy. The study excluded 22 women who had undergone LPT hysterectomy with dissection of the pelvic and aortic lymph nodes (Fig. 1).
A gynecologic oncology expert performed the entire surgical operation. Each and every person received TH and BSO. Moreover, 92 patients had PLND procedures. PLND involves the removal of lymphatic tissues around external and common iliac vessels and from the obturator fossa. The updated FIGO staging systems from 2009 and 2023 were used to classify patients with EC. Evaluations were conducted based on patient clinical and pathological features, age, histological type, grade, myometrial invasion status, tumor size, LVSI, involvement of the ovaries and cervix, and existence of lymphatic metastases.
SPSS (Statistical Package for Social Sciences) 20 for Windows (IBM SPSS Inc., Chicago, IL) was used to conduct the statistical evaluation. The Kolmogorov-Smirnov test was employed to assess whether the measured data conformed to a normal distribution. The variables‘ n and percentage values were provided. Categorical data were compared using Fisher‘s Exact Test. The study employed logistic regression analysis to ascertain the impact of the participants‘ parameters on survival and recurrence. Results of statistical analysis were deemed significant if the p-value was less than 0.05.
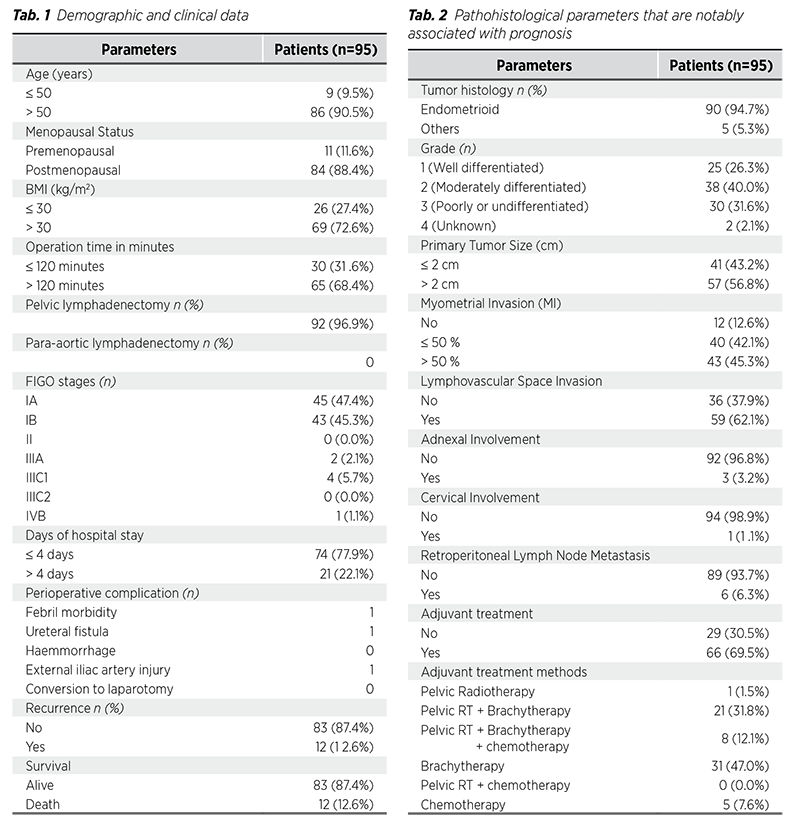
The study included 95 women with endometrial carcinoma who had laparoscopic hysterectomy at the Department of Oncology of Azerbaijan Medical University from 2018 to 2024. Ninety-five patients were treated via MIS and observed for endometrial cancer as long as the study period lasted. Twenty-five (26.3%) women had grade I, 38 (40.0%) had grade II, and 30 (31.6%) had grade III endometrioid endometrial tumors. Tumor grade of 2 patients was unknown. The most common performed surgical procedure was laparoscopic hysterectomy+BSO+BPLND in ninetytwo (96.9%) patients. The number of premenopausal and postmenopausal women were 11 (11.6%) and 84 (88.4%), respectively. Sixty-nine (72.6%) of the patients had a BMI greater than 30. Of the patients, 88 (92.7%) had FIGO stage I tumors, and 57 (56.6%) had tumors larger than 2 cm. Of the patients, myometrial invasion was less than or equal to 50% in 40 (42.1%). Additionally, myometrial invasion was greater than 50% in 43 (45.3%), and only 12 (12.6%) had no observable myometrial invasion. Six patients (6.3%) had lymph node metastases, 59 patients (62.1%) had LVSI, 3 patients (3.2%) had adnexal involvement, and one patient (1.1%) had cervical involvement. There was no convert from laparoscopy to laparotomy during the surgical process.
Duration of procedure more than 120 minutes in laparoscopy was 65 (68.4%). Additionally, shorter hospital stay (≤4 days) was 74 (77.9%). In the study population, the longest observation duration was 77 months. The recurrence was recorded in twelve patients (12.6%). During the follow-up period, survival data was gathered and revealed 87.4% (n=83) survival rate for the procedure. Tables display all demographic, clinical, and histopathological characteristics (Tab. 1, 2).
The purpose of this study was to look at how MIS affected surgical and oncological prognosis results in uterine cancer patients. The findings show that laparoscopic procedures have adequate exposure, efficacy and safety in patients with uterine-confined endometrial cancer.
This study also demonstrated the benefits of MIS for postoperative recovery. Less ileus and bleeding risk, as well as fewer surgical infections were observed in our patient group who underwent LPS. Even though the somewhat longer procedure time, this led to early hospital discharge. The median operating times for laparotomy (130 minutes) and laparoscopy groups (204 minutes) were different in the LAP2 study (12). Furthermore, Janda M, et al. have found that the duration of LPS was statistically substantially longer (13). Our study results are similar to mentioned of above two prominent randomized controlled trials (12,13). Studies that have already been conducted on the use of laparoscopy in patients with EC have found that the treatment can be done minimally invasively and that recovery is quicker than with open procedures (14). Because of the advantages of minimally invasive techniques, laparoscopy is being employed in gynecologic oncology more frequently (15). With technical experience, high BMI in patients should not be considered a deterrent for procedural involvement. For obese women, laparoscopy should be the first option to decrease the incidence of wound infection. While there is a continuing discussion over doing lymph node dissection for early-stage EC, all research patients underwent pelvic lymph node dissection. The amount of retroperitoneal lymph nodes removed from patients in our study was similar to the literature, and it was at the same time adequate for staging (16). As said the current study‘s complication rates, patients that underwent laparoscopy showed lower rates of febrile morbidity, ileus, and urinary tract infections. Laparoscopy was linked to a noticeably decreased rate of postoperative complications, based on randomized controlled trials that assessed the whole perioperative complications (17).
There are several limitations to our study. The study‘s first drawback is the heterogeneous population and its retrospective nature. Due to the retrospective nature of the study, it is prone to selection bias and confounding factors, which may affect the validity of the findings. Additionally, results could have been impacted by modifications in surgical methods during the course of the 6-year study. The study‘s modest sample size and single-center design constitute its second drawback. Since the study is singlecenter, the results cannot be generalized. Another limitation is the exclusion of patients undergoing laparotomy procedures. This may lead to a selection bias as different baseline characteristics of these patients may affect the results. It is important for the consistency of the results that all procedures are performed by a single gynecologic oncologist. However, this also limits the applicability of the findings to other settings with different levels of surgical expertise. Another limitation of our study is that our study results cannot be compared because there was no control or laparotomy group. Additional restrictions on the patient population include the exclusion of patients who cannot be reached by phone from the study, and incomplete records found in files scanned in the hospital registration system. Therefore, greater sample sizes and multicenter, randomized controlled investigations should validate the findings of this investigation. Further research is necessary to evaluate the initial experiences of surgeons using MIS in gynecologic oncology, especially for young surgeons in underdeveloped countries. Even though meta-analyses and randomized control trials have been conducted, evaluation of the benefits of laparoscopic surgery in the management of endometrial carcinoma is still needed.
According to our study, laparoscopy plays an important role in the treatment of uterine-confined endometrial carcinoma for surgeons who have experience with commencing gynecologic cancer surgery. In these cases, LPS may be conducted with a comparable level of lymph node dissection to laparotomy, a shorter hospital stay, and fewer postoperative infections. We believe that our results will be a valuable reference in clinical settings. However, confirming the results of our study with prospective randomized controlled studies and especially analyzing the results of control or laparotomy groups will strengthen our study results. MIS should be encouraged in the treatment of endometrial cancer nowadays in all low-income countries.
Acknowledgements: We would like to acknowledge the www.makaletercume.com for their outstanding scientific proofreading and editing services that was provided for this manuscript.
Competing interests: The authors have no conflict of interest in this study.
Data availability statements: The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics statement: Approval from the local ethics committee of the Oncological Department of the Azerbaijan Medical University (2021-12-06/N238).
Funding: This study was not funded by any person or organization.