










 Official publication of the Czech Society of Ultrasound in Obstetrics and Gynecology.
Official publication of the Czech Society of Ultrasound in Obstetrics and Gynecology.

Introduction and Objective: Folate (vitamin B9) supplementation during pregnancy to prevent neural tube defects (NTDs) in the fetus is a widely accepted standard of care. Folate is also vital for maintaining proper brain function. Additionally, folic acid works in conjunction with vitamin B12 ro produce red blood cells and aids in the proper functioning of iron in the body. The most effective form of folate supplementation remains a topic of ongoing discussion. This review aims to collect information on the effects of folic acid and its derivatives on reproductive health.
Vitamins are essential organic molecules needed by the body in small quantities to perform specific functions. Most of them are not produced in sufficient amounts by the body so they must be obtained through the diet. Folic acid (pteroylmonoglutamic acid), the synthetic, water-soluble form of vitamin B9, is commonly found in supplements and fortified foods, whereas folate is the naturally occurring form present in various foods. Folic acid is inactive in the human body and must be converted by the liver into the active compound 5-methyltetrahydrofolate (5-MTHF).
Vitamin B9 and its derivatives play a crucial role for DNA synthesis and repair, which are critical processes during the rapid cell division and growth that occur in early fetal development (1). However, folic acid can pose health risks in certain situations, such as in cases of megaloblastic anemia, where it may mask the condition caused by a vitamin B12 deficiency. It may also be problematic for individuals with reduced hepatic conversion of folic acid due to genetic variants or certain medications. These risks can be reduced by using 5-MTHF instead of folic acid. 5-MTHF plays a crucial role as a methyl donor in various metabolic processes, including the conversion of homocysteine to methionine, the synthesis of glycine from serine, and the production of DNA precursors.
The process of converting folates supplied with food or in the form of dietary supplements is one of the most important metabolic pathways occurring in the cell. This pathway affects the proper functioning of many systems and organs, including in particular the cardiovascular, circulatory and nervous systems.
In the human body, folate is absorbed in the mucosal epithelial cells of the enterocytes. The folate found in food primarily exists in the polyglutamate form, which must be converted by enzyme glutamate carboxypeptidase II (GCPII) to the monoglutamate form before it can be absorbed. This enzyme is predominantly located in the brush border of the proximal jejunum. Once in the monoglutamate form, folate is transported across the apical membrane of enterocytes mainly through the proton-coupled folate transporter. Within enterocytes, folate is converted into 5-methyl-THF and subsequently transported into the portal vein through a multidrug resistance-associated protein, where it then circulates in the bloodstream. Cells absorb that from the blood through the reduced folate carrier or via receptormediated endocytosis of folate receptors (FRs) (2).
Inside cells folylpoly-gammaglutamate synthetase converts folate into polyglutamate forms. The biological activity of folic acid depends on the enzyme dihydrofolate reductase (DHFR), which is produced in the liver. To become biologically active, folic acid must be reduced by DHFR first to dihydrofolate and then to tetrahydrofolate (THF). THF is subsequently converted to its active form, 5-methyl-THF (5-MTHF) (3,4).
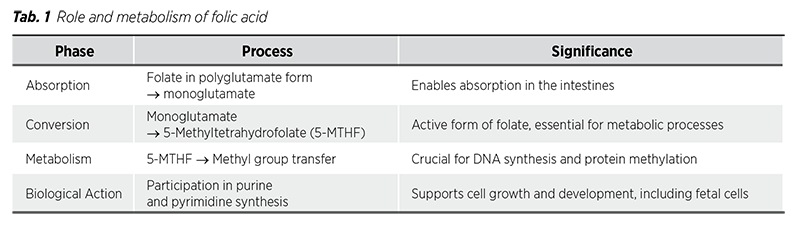
5-methyl-THF serves as a carrier for providing a singlecarbon unit essential for the synthesis of purines and pyrimidines. Tab. 1 summarizes the phases and processes involved in folic acid metabolism. Additionally, in the methionine synthesis pathway, 5-MTHF donates a methyl group to a vitamin B12 coenzyme, allowing homocysteine to be converted into methionine, which can then be further processed.
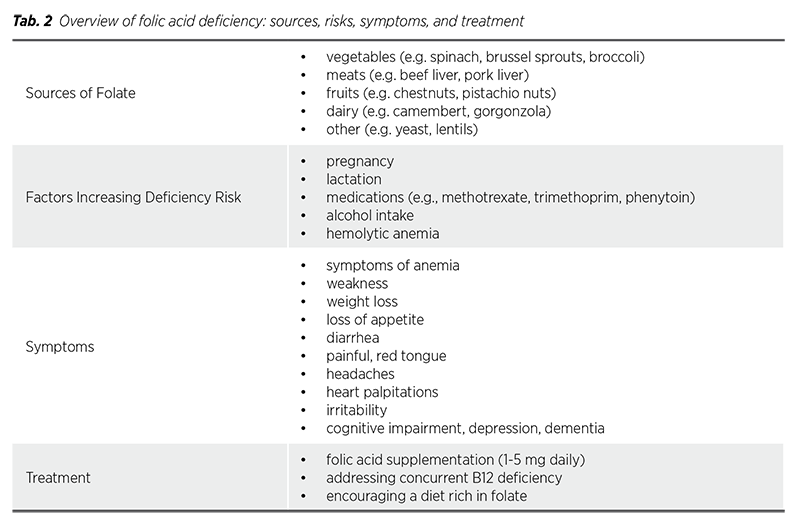
Humans are unable to synthesize vitamin B9, making it essential to obtain this nutrient from dietary sources. Folate is naturally present in a variety of foods, including vegetables like spinach or broccoli, as well as meats like beef or pork liver. To address potential deficiencies, many countries have implemented mandatory food fortification with folic acid to ensure adequate dietary intake. However, it can be challenging for many individuals to meet the recommended daily intake of vitamin B9 through diet alone. Additionally, the bioavailability of food-derived folates can be reduced due to oxidation caused by heat, light, or metal ions during cooking (5). Because of that, this deficiency remains common, particularly in individuals who do not take supplements. The demand for vitamin B9 increases during pregnancy and lactation, making it even more critical to address this need. Certain medications, such as methotrexate and trimethoprim, as well as alcohol consumption, can further increase the risk of deficiency (6). Folate deficiency can be asymptomatic but may also present with symptoms such as anemia, loss of appetite, diarrhea and a painful tongue. Other signs include weakness, headaches, heart palpitations, irritability, and behavioral disorders like cognitive impairment, depression, and dementia (6).
When evaluating patients for folic acid deficiency, it is crucial to also check vitamin B12 levels, as both conditions can produce similar findings in a complete blood count. Laboratory results often reveal macrocytic anemia with an increased mean corpuscular volume (MCV) greater than 100. Testing serum levels of vitamin B12 and folate can help differentiate between these two conditions (6).
All patients diagnosed with folate deficiency should be provided with supplemental folic acid to correct it. Generally, a dose of 1 to 5 mg daily is sufficient for treatment. For individuals who also have low levels of a vitamin B12, it is crucial to address it concurrently, as treating folate deficiency alone will not alleviate the neurological symptoms caused by B12 deficiency. Without appropriate B12 supplementation, these symptoms could worsen and lead to permanent neurological damage. Additionally, patients should be encouraged to consume a diet rich in fruits and vegetables (6).
Tab. 2 provides a summary of folate sources, factors increasing deficiency risk, symptoms of deficiency and recommended treatment strategies.
Proper folate metabolism leads to the methylation of DNA and proteins through the remethylation of homocysteine to methionine, followed by conversion to S-adenosylmethionine (SAM), the main donor of methyl groups. This process is essential for embryo and fetal development, influencing gene expression and cell differentiation. The body undergoes over 100 methylation reactions, including those involving DNA, RNA, histones, and phospholipids (7). Folate metabolism is assessed via the SAM/SAH ratio, with a decrease indicating methylation issues (7). Insufficient folate intake can result in fetal defects, adult diseases (8), and is linked to Down syndrome and autism (9).
Research at the end of the 20th century confirms the crucial role of folates in reducing the risk of neural tube defects (NTDs). Numerous randomized and cohort studies have shown that supplementation with 400-800 mcg of folic acid significantly lowers the risk of these defects (10-12). Since neural tube closure occurs by the 28th day (13), it is essential to ensure adequate folate levels in the mother during the preconception period. Studies have demonstrated that taking 400 micrograms of 5-MTHF or folic acid for 8-12 weeks is sufficient to achieve proper folate saturation, while a dose of 0.8 mg can achieve this effect in about 4 weeks (7).
It’s strongly advised that all women who have the potential to become pregnant, particularly those actively planning or attempting to conceive, should take a daily supplement of 400 mcg of folic acid (14). This practice should be continued consistently until reaching the 12th week of pregnancy to ensure adequate folate levels for optimal fetal development (14).
For individuals with a history of a pregnancy affected by a neural tube defect (NTD), there is an increased risk of having another such pregnancy. To mitigate this risk, it is advised that such individuals initiate a high-dose folic acid supplementation regimen of 4,000 mcg daily, beginning at least one month before conception and continuing through the first three months of pregnancy (15). In addition to high-dose supplementation, individuals with a history of NTDs should be encouraged to increase their dietary intake of folate through food sources rich in this essential nutrient (14). For those who are not planning a pregnancy, the general recommendation remains at a daily intake of 400 mcg of folic acid (15).
MTHFR is a highly polymorphic gene in the general population, with numerous alterations identified in it. To date, 35 rare mutations, polymorphisms, and nine common variants have been reported, with C677T and A1298C being the most prevalent. Approximately 40% of people worldwide present some form of MTHFR polymorphism (5). This genetic variation can reduce MTHFR enzyme activity, with the altered one functioning at about 55% to 70% efficiency compared to the normal version (5). This reduction can impair the conversion of dietary folates and folic acid into 5-MTHF, potentially increasing the risk of various adverse outcomes in pregnant women with polymorphisms. Elevated homocysteine (Hcy) levels, resulting from folate deficiency, are also recognized as independent risk factors for many pregnancy-related disorders.
Supplementing with the active form of folate, 5-MTHF, bypasses the impaired folate metabolism associated with MTHFR polymorphisms, enabling direct absorption and utilization of folate for biological functions. Several studies have examined the effects of 5-MTHF supplementation in individuals with these genetic variations (5). For example, Prinz-Langenohl et al. (16) demonstrated that MTHFR gene polymorphism doesn’t affect 5-MTHF supplementation. Consequently, using 5-MTHF instead of folic acid is strongly recommended for people with MTHFR polymorphisms.
Individuals with fertility challenges may experience low folate availability, often linked to MTHFR enzyme polymorphisms. In recent years, there has been a growing body of research on the relationship between folates and fertility issues, indicating that 5-MTHF is a more effective alternative to folic acid. Several studies have investigated the impact of 5-MTHF supplementation in couples facing infertility, particularly those who had previously been using high doses of folic acid (17,18). Furthermore, a considerable proportion of these couples carried the MTHFR C677T isoform. Remarkably, many of them achieved pregnancy after switching to 5-MTHF (17,18). Although these results are encouraging, the studies were conducted with a relatively small sample size, indicating the need for more extensive research.
What’s more, as a result of mutations in the MTHFR gene, homocysteine can accumulate in the blood, leading to elevated levels. Extended exposure to high homocysteine levels can damage blood vessels, increasing the risk of blood clots and development of cardiovascular diseases, including stroke (5). Since healthy blood vessels and adequate folate levels are crucial for fertility and pregnancy, elevated homocysteine can make it more challenging to conceive and sustain a pregnancy. Research by Mazza et al. (19) showed that a combination of 400 μg of 5-MTHF with vitamins B6 and B12 was more effective at reducing serum homocysteine levels compared to traditional high-dose folic acid supplementation (5 mg/day). Additionally, Litynski et al (20). showed that 5-MTHF supplementation resulted in a significant, prolonged reduction in Hcy levels up to six months after discontinuing treatment in homozygous TT individuals.
Based on these findings, it is advisable for individuals with MTHFR polymorphism - especially couples facing fertility issues to take special consideration of their supplementation choices. This advice is especially pertinent for those where one partner carries one of the two primary MTHFR isoforms, either C677T or A1298C. For these individuals, choosing 5-MTHF over the more commonly used folic acid might lead to better outcomes. Additionally, in countries lacking folate fortification programs, using the natural form of 5-MTHF could be a more effective alternative to folic acid.
Folic acid plays a vital role in human health, particularly in DNA synthesis and repair, which are critical processes during early fetal development. A deficiency in folate can lead to a range of serious health complications, such as anemia. In pregnant women, inadequate folate levels can result in significantly increased risk of neural tube defects (NTDs) in the developing fetus.
In individuals with MTHFR gene polymorphisms, which can hinder the body’s ability to metabolize folate effectively, folate levels may be inadequate. As a result, supplementation with 5-MTHF is recommended instead of traditional folic acid. 5-MTHF, being the active form of folate, bypasses the need for metabolic conversion, thus ensuring more efficient absorption and utilization, and ultimately improving folate levels. Moreover, 5-MTHF supplementation has shown promise in enhancing outcomes for couples experiencing fertility issues, particularly those with MTHFR polymorphisms.
Elevated homocysteine levels, which often arise from MTHFR polymorphisms, are associated with an increased risk of cardiovascular diseases. This connection further emphasizes the necessity of maintaining optimal folate levels to support overall well-being.
Ongoing research and personalized medical recommendations are crucial. Recent studies advocate for the use of 5-MTHF over folic acid, highlighting the importance of tailored approaches to supplementation and the need for continued investigation into optimal strategies for addressing individual nutritional needs.