










 Official publication of the Czech Society of Ultrasound in Obstetrics and Gynecology.
Official publication of the Czech Society of Ultrasound in Obstetrics and Gynecology.

Background: Cervical cancer (CC) is the fourth most common cancer in women. Except for HPV, other factors as miRNAs play an important role in carcinogenesis. There were found specific patterns of miRNA, which make them possible tumor biomarkers of CC.
Methods: We searched for studies published in the English language in the PubMed database from 2014 to 2021. The following medical subject headings were used: (“miR-21” [Title/Abstract]), (“miRNA-21” [Title/Abstract]), (“microRNA-21” [Title/Abstract]), (“cervical cancer” [Title/Abstract]), (“biomarker” [Title/Abstract]). Only studies that were published as full-text journal articles were used and carefully checked. According to keywords, 35 studies were reviewed. Among them, 2 studies demonstrated overexpression of miR-21 also in other malignancies, and 2 studies described new potential tools for identifying miR-21 as a cancer biomarker. Six review studies were excluded. The last 25 works were reviewed.
Results: Totally, in all articles in our table, it was taken 367 samples of CC tissue samples, 45 cervicovaginal lavages, and 25 blood samples in patients with confirmed CC. In all samples of patients with CC, overexpression of miR-21 was confirmed. We reviewed 12 other studies that confirm the role of miR-21 in the development of cervical lesions, but also in the spread of lymph node metastasis and chemoradiotherapy resistance.
Conclusion: Demonstrated overexpression of miR-21 in CC cells and recognizing the signaling pathways affected by this molecule suggests using the miR-21 as a suitable non-invasive biomarker of CC.
Cervical cancer (CC) is the fourth most common cancer in women worldwide after breast, colorectal, and lung cancer, respectively. According to the Global Cancer Observatory (2021), 604,127 new cases of CC were diagnosed, and 341,831 patients died on CC in 2020 worldwide, respectively (1). Histologically, three main forms of CC are known. Squamous cell carcinoma represents about 70% of all cases, while this can be divided to moderately differentiated non-keratinizing large cell type, well-differentiated squamous cell carcinoma and poorly differentiated small cell type. Cervical adenocarcinoma is identified in 20-25% of all CC cases and about 5% of cases are represented by others histological types like adenosquamous or verrucous carcinoma (2). Cervical carcinogenesis closely correlates to the presence of Human Papillomavirus (HPV) infection in women and is also associated with risk factors as multiparity, early coitarche, multiple sexual partners, smoking or low socioeconomic status (3). However, only these factors are not enough to start the mentioned transformation. Other factors, such as microRNAs (miRNAs), play a role in carcinogenesis, too.
MiRNAs are single-stranded ribonuclear acids consisting of 18 to 25 base pairs. They are transcribed from DNA like other RNAs but do not encode any proteins. They affect gene expression binding to mRNAs and influence their translation. More than 2000 miRNAs have been described in the human body, and it is thought that these particles affect the expression of about one-third of the human genome. MicroRNAs can occur intracellularly, also as a freecell associated with an Argonaute protein or as a part of extracellular vesicles (EV) (4).
MicroRNA biogenesis consists of five steps in the vertebrates. The first step is processed in the nucleus. A primary transcript – miRNA precursor – pri-miRNA is formed from the genome of RNA by RNA polymerase II. In the intergenic region, miRNA has its promoter; it uses the host gene promoter in the intron region. Rarely, it can be synthesized by forming the opposite complementary strand by RNA polymerase III. In the second step, the pri-miRNA is modified by Drosha – double-stranded RNA endoribonuclease, forming a “steam loop” at one end of the double- stranded RNA. Formed pre-miRNA is transported from the nucleus to the cytoplasm (5).
In the cytoplasm, the double-stranded pre-miRNA is cleaved near the terminal loop by Dicer endoribonuclease. Dicer belongs to the ribonuclease family III, like Drosha. Subsequently, one of the strands of the miRNA duplex binds to the Argonaute protein as an essential component of the RNA-induced silencing process (RISC) in the process of RNA interference, inhibiting gene expression or translation by neutralizing RNA. Thus, modified miRNAs can further:
a) be imported into the nucleus, where they may positively or negatively regulate transcription,
b) bind to the 5’ end of mRNA in the cytoplasm with the positive or negative effect on posttranscriptional regulation,
c) the miRNA strand after contact with the RISC can bind to the 3’ end of the mRNA and cause its degradation or inhibition of translation,
d) the miRNA can be released extracellularly either as a part of the extracellular vesicles or bound to the Argonaute protein. Significantly, only extracellular vesicles-miRNAs reportedly have a function in communicating between cells and play a role in various biological processes (5).
The first evidence of microRNA role in the process of carcinogenesis dates to 2002. Calin et al. (6) provided that loss of miR-15a and miR-16-1, caused by deletion of chromosome 13q14, play important role in chronic lymphocytic leukemia carcinogenesis (6). Dysregulation of microRNAs in carcinogenesis may leads to loss of their function as tumor suppressors and on the other hand can also induce oncogene function (7). For example, miR-21 play role in different tumor types included colorectal cancer (8) and breast cancer (9).
Progression of precancerous lesions of the cervix to the invasive cervical carcinoma has been repeatedly proven to be in relation with changes in microRNAs expression levels (10). On the one hand, the microRNAs can act as tumor suppressors, for example as miR-29, which by influencing Suppressors of Cytokine Signalling 1 (SOCS1) or Smad nuclear interacting protein 1 (SNIP1) in work Gong Y. et al. (11) and Chen Y. et al. (12), has led to the suppression of cervical carcinogenesis. On the other hand, there are known miRNAs whose up regulation is associated with carcinogenesis. Hsa-miR-21-5p, generally known as miR-21, is one of the most highly expressed miRNAs in mammals and is up-regulated in many cancers (10). Many literature sources also point to the upregulation of miR-21 in the process of cervical carcinogenesis, which led us to the idea of gathering articles dealing with this issue and then evaluating the results and expressing the premise of the suitability or non-suitability of using the miR-21 as a cervical cancer marker.
One way how to influence cell proliferation in CC is to regulate miR-21 expression. It acts on the development of cancer through several signaling pathways:
1. miR-21 enhances cell proliferation and inhibits cell apoptosis via TNF-α: miR-21 expression levels have been shown to affect tumor necrosis factor α (TNF-α) expression in alveolar macrophages. The TNF-α is produced by various cell types such as T and B lymphocytes, fibroblasts, macrophages, monocytes, and it is an important mediator of inflammation and apoptosis. The TNF-α interacts with its TNF 1 and 2 receptors and stimulates processes such as cell differentiation, cell migration, angiogenesis, apoptosis, necrosis, and activation of the cellular immune response. miR-21 has been shown to up-regulate TNF-α through an unknown effector on human epithelial cells (HeLa), cervical cancer cells (13).
Xu et al. (13) found miR-21, miR-21 inhibitor, and control miRNA were in vitro transfected into HeLa cervical carcinoma cells. Within 36 and 48 hours after transfection, a significant increase of TNF-α mRNA expression was demonstrated (13). TNF-α binding to its TNFR1 receptor activates the process of cell apoptosis. In contrast, cell proliferation is activated by the binding of TNF-α to the tumor necrosis factor receptor 2 (TNFR2), followed by up-regulation of nuclear factor kappa B (NF-κB). Up-regulation of NF-κB leads to inhibition of caspase 3, which is the protein responsible for cell apoptosis and activation of c-Jun N-terminal kinase (JNK) leading to cell proliferation (14).
2. miR-21 enhances tumorigenesis via PI3KAkt- mTOR signaling pathway: another possibility of how miR-21 affects the process of cervical carcinogenesis is its entry into the phosphoinositide 3-kinase (PI3K) / protein kinase B (PKB, also known as Akt) / mammalian target of rapamycin (mTOR) signaling pathway via the tumor suppressor PTEN (Phosphatase and Tensin Homolog). PI3K/Akt and mTOR signaling pathways are both crucial to many aspects of cell growth and survival, in physiological as well as in pathological conditions (15).
PI3K/Akt: After activation of PI3K via the receptor for growth factor in the cell membrane, a second messenger – phosphatidylinositol-3,4,5-triphosphate (PI3,4,5-P3) is formed in the cell. PI3,4,5-P3 activates 3’ phosphoinositide-dependent kinase 1 (PDK1) and Akt. Activated Akt and PDK-1 can phosphorylate many downstream proteins to regulate cell growth, motility, survival, and metabolism (16). The signal is transmitted from the cellular receptor via PI3K/Akt to mTOR, leading to increased synthesis of proteins involved in tumorigenesis, such as cyclin D1 – a protein required for progression through the G1 phase of the cell cycle or Hypoxia-Inducible Factors – protein with impact on the expression of genes, which ensure cell survival in hypoxic conditions (e.g., Vascular endothelial growth factor (VEGF)). PTEN is a negative regulator of the pathway described above. Li et al. (17) state that PTEN is a downstream target of miR-21 and that the miR-21 inhibitor inhibited cell proliferation, induced G1 arrest, and promoted cell apoptosis by modulating the PTEN/Akt pathway (17).
3. Programmed Cell Death 4 (PDCD4) is known as a tumor suppressor gene that inhibits cell growth, tumor invasion, metastasis, and induces cell apoptosis. Down-regulation of PDCD4 leads to an increase of HeLa cell growth. The PDCD4 protein synthesis is inhibited by microRNAs, including miR-21 (18).
4. miR-21 enhances cell proliferation, inhibits apoptosis, and causes malignant transformation cells via Ras-Raf-Mek-Erk signaling pathway: miR-21 may also enter the Ras-Raf-mitogen-activated protein kinase (MAPK also known as MEK) – extracellular signal-regulated kinases (ERK) signaling pathway. The Son of Sevenless (SOS) – a group of several genes encoding the “guanine nucleotide exchange factor” that exchanges GDP for GTP on Ras and thus Ras becomes activated – is activated by activation the tyrosine kinase receptor. Activated Ras activates Raf kinase, which activates MEK by phosphorylation. It again activates MAPK, originally called ERK, by phosphorylation. ERKs are kinases responsible for the inhibition of apoptosis, cell mitosis, and malignant cell transformation. miR-21 enters the signaling pathway by inactivation of the p120- Ras GTPase activating protein (RasGAP) – a negative regulator of this signaling pathway. miR-21 drives tumorigenesis via inhibition of negative regulators of the Ras/MEK/ERK pathway and inhibition of apoptosis (19).
5. Deregulation of extracellular matrix homeostasis is associated with tumor tissue growth and metastasis spread. Matrix metalloproteinases and their inhibitors, including the tissue inhibitor of metalloproteinase 3 (TIMP3), are involved in maintaining homeostasis. TIMP3 by inhibiting metalloproteinases reduces the ability of tumor cells to invade and migrate. miR-21 promotes the proliferation, viability, and migratory and invasive activities of cervical cancer cells through targeting TIMP3 (20).
6. The accumulation of long non-coding RNAs (lncRNAs) also plays a role in cell carcinogenesis. As a part of the signaling pathway mentioned above, the mTOR ensures the accumulation of GAS5 growth arrest-specific 5 (GAS5) lncRNA in the cell. Overexpression of GAS5 lncRNA in gastric cancer inhibits miR-222 expression and thus suppresses cell proliferation in this tumor. In contrast, miR-21 in CC causes down-regulation of GAS5 lncRNA, resulting in increased cell proliferation and tumor cell invasion. In patients with cervical cancer, the GAS5 expression level was associated with the FIGO stage, metastatic parameter, clinical staging, and overall survival (21).
We searched for studies published in the English language in the PubMed database from 2014 to 2021. The following medical subject headings were used: (“miR-21” [Title/Abstract]), (“miRNA-21” [Title/Abstract]), (“microRNA-21” [Title/Abstract]), (“cervical cancer” [Title/Abstract]), (“biomarker” [Title/Abstract]). Studies whose titles and abstracts did not meet selection criteria were excluded from this review. Only studies that were published as full-text journal articles in English were used and carefully checked. Review studies were excluded. According to keywords, 35 studies were reviewed. Among them, 2 studies demonstrated overexpression of miR-21 also in other malignancies, and 2 studies described new potential tools for identifying miR-21 as a cancer biomarker. Six review studies were excluded. Finally, the last 25 articles were reviewed.
Based on previous reports, we chose to investigate the role of miR-21 in the carcinogenesis of CC. According to keywords, we chose manuscripts from the years 2014 to 2020, which described changes of expression miR-21 in samples of patients with confirmed CC. For identification of miR-21 and the level of its expression in the studies mentioned above, there were mostly used kits containing Trizol reagent. The concentration of this RNA was verified by spectrophotometer and its quality by electrophoresis. Isolated RNA was most often converted to complementary cDNA by reverse transcriptase (RT), and for the subsequent detection, quantitative polymerase chain reaction (PCR), real-time PCR, or microarray were used. In all of these studies, overexpression of miR-21 has been demonstrated, either in the serum or in tumor tissue of patients with CC. In some papers, a relation between miR-21, precancerous lesions, and the presence of HPV was also described. There were taken 367 samples of CC tissue samples, 45 cervicovaginal lavages, and 25 blood samples in patients with confirmed CC. All these studies show overexpression of miR-21 in patients with CC (Tab. 1).
MicroRNAs are released into the circulation after apoptosis of the cells or during their necrosis, but they are often excreted by the tumor cells as well. Their levels may indicate the degree of cancer development, whether at the level of the tumor itself or its metastasis. High stability of miRNAs was demonstrated in multiple cancer samples (22). Extracellular miRNAs are potential biomarkers for many diseases (23). Up-regulation of miR-21 has been confirmed in many oncological diseases, either solid tumor origin or leukemic origin, and its function has been the subject of many studies in the past decade.
Han et al. (24) described the localization of the miR-21 gene in the fragile region of the chromosome (FRA1 7B), which is one of the regions of HPV 16 integration in the host genome. It is thought that the integration of HPV 16 near the miR-21 gene may affect the development of CC, and thus miR-21 is involved in the process of cervical carcinogenesis (24).
Liu et al. (25) confirmed increased miR-21 expression in cervicovaginal lavage exosomes of patients with confirmed CC (25). Park et al. (26) also confirmed the presence of miR-21 in CC tissue cells, and thus it’s potential to become a biomarker of this disease. This study has shown that miR-21 expression is not only increased in CC cells in which high-risk HPV (hrHPV) has been implicated, but also independently of the presence of HPV infection (26). Zamani et al. (27) did not show significant deregulation of miR-21 or miR-29 in cervical tissue cells without tumor changes with confirmed HPV infection in comparison to the control group – without HPV infection and cervical tumor lesion. However, the deregulation of these microRNAs in the cervical tumor lesion tissue cells has already been significant, suggesting the possibility of using miR-21 as a marker of CC in its early stages (27).
Yuan et al. (28) again confirmed increased miR-21 expression in CC tissue with present HPV infection. Moreover, this study monitored levels of VEGF in patients with malignant cervical lesions and explained the mechanism of action of miR-21 by inhibiting PTEN gene expression as is described above. It also demonstrates the inhibition of the PTEN gene leading to up-regulation of VEGF expression in breast tumor cell samples of dogs, and thus improves aggressiveness of tumor tissue growth by improving vascular supply (28). This raises the question of whether the VEGF levels are also elevated in patients with CC with the presence of HPV infection. The authors demonstrated increased survival in patients with CC lesions whose miR-21 and VEGF levels were low. Zhu et al. (29) demonstrated up-regulation of miR-21a and down-regulation of miR-34a in CC tissue. They pointed out the possibility of using these ribonuclear acids as markers of CC progression and potential molecular targets in arresting CC’s development (29).
Zamani et al. (30) showed an increased miR-21 expression in patients with CC. However, this article also pointed to a lack of demonstrated correlation between mi-R21 expression and the presence of HPV infection in the CC cells (30). Okoye et al. (31) observed the level of miR-21 expression in serum and cervical tissue cells at various stages of cervical lesion development – from cervicitis through ASCUS, LSIL, HSIL to squamous CC. They observed an approximately 2-fold increase in miR-21 expression in cervical inflammatory lesion cells against healthy, non-inflammatory tissue (31). Their results were in correlation with a previous study of Bumrungthai et al. (32). A 1.5-fold increase in miR-21 levels between LSIL and HSIL lesions was also demonstrated, which
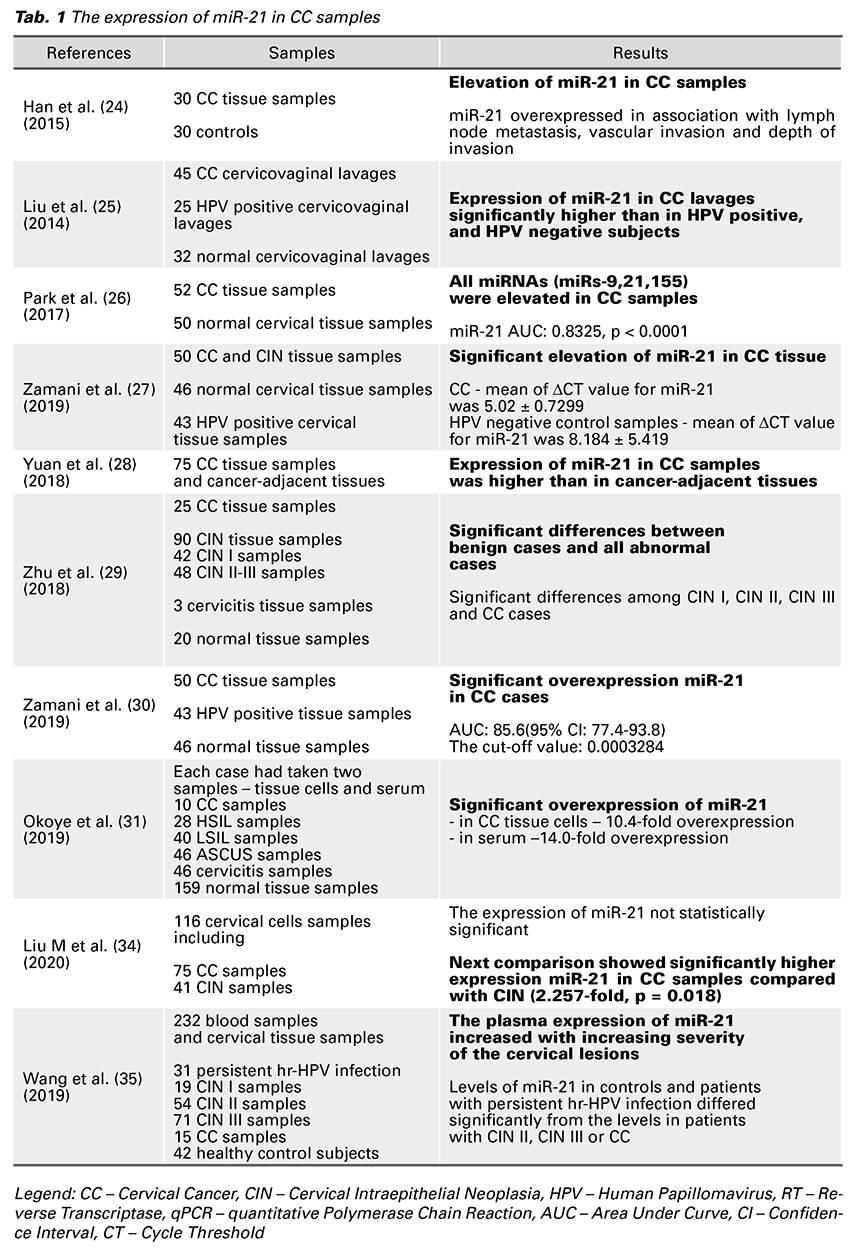
was consistent with Gocze et al. (33). These facts favorize miR-21 as a suitable candidate for a non-invasive assessment of the progression of cervical transformation.
Liu M et al. (34) compared changes in miR-21 expression between the CIN stage and CC as well as changes in the expression of this RNA in CC cells with evidence of HPV 16, 52, and 58 infections. miR-21 expression was 2.266 times higher in CC cells than in CIN lesion cells. In the same comparison of lesions in which HPV 16 infection was demonstrated, the difference in expression in CC tissue versus CIN was 3.618 times greater. The difference in miR-21 expression between CC and CIN lesions, both with evidence of HPV 52 or HPV 58 infection, was not statistically significant (34). These facts pointed to the possibility of using miR-21 as a marker to distinguish between CIN lesions and a CC, especially in the presence of HPV 16 infection.
Wang et al. (35) described overexpression of miR-21 in blood samples of patients with CIN2, CIN3, and CC in comparison with normal controls and patients with persistent hr-HPV infection. There was no significant difference between the normal control group and patients with persistent hr-HPV. In cervical tissue samples were not differences in miR-21 expression of CIN1, CIN2, and CIN3. The levels of miRNA in plasma and cervix tissue were consistent (35).
Other reviewed studies also confirmed overexpression of miR-21 in samples taken from patients with confirmed CC. Zhang et al. (20) examined 60 cervical cancer tissue samples and 60 serum samples. This study described the impact of miR-21 on the expression of TIMP3. miR-21 promoted the proliferation, viability, and migratory and invasive activities of cervical cancer cells through targeting TIMP3. The role of TIMP3 in cervical carcinogenesis was described above (20). Dai et al. (36) collected 82 cervical cancer specimens. This study indicates that Kruppel like factor 4 (KLF4) and Estrogen receptor 1 (ESR1) are downregulated by the upregulated miR-21 and miR-16 in cervical cancer, respectively. They elucidated that miR-21 overexpression might lead to the pathogenesis of cervical cancer by inhibiting the expression of KLF4 and ESR1 in cervical tissues (36).
Liu Q et al. (37) described a group of 65 patients who underwent cisplatin-based chemotherapy and radiotherapy. In advanced cervical cancer samples, the elevation of miR-21 expression was observed, while decreased levels of Sma and Mad proteins from Caenorhabditis elegans and Drosophila (SMAD) 7 were confirmed in cancer tissue from patients resistant to the therapy. TGF-beta cytokines employ SMAD proteins as the intracellular mediator of signaling. The signaling pathway where miR-21 enhances cell proliferation and inhibits cell apoptosis via the TNF-α we mentioned above. This work provided that decreased miR-21 may improve drug resistance targeting Smad7 in CC and suggested that the miR-21/ Smad7 pathway may be an effective target for drug resistance in cervical cancer treatment (37, 38).
Yao et al. (39) described the positive association of cisplatin-induced apoptosis in cervical cancer cells and GAS5 overexpression. Expression levels of the GAS5 are inversely associated with malignancy. This work proved that GAS5 suppresses miR-21 expression, which also points to the role of miR-21 in CC tumorigenesis. How miR-21 in CC causes down-regulation of GAS5 lncRNA we mentioned above (39).
Gómez-Gómez et al. (40) showed that the expression of miR-21 was up-regulated by the HPV16 E7 oncoprotein. Overexpression of miR-21 leads to CC tumorigenesis through suppression of PTEN expression, as we described above. This work pointed to miR-21 as a significant factor in cervical tumorigenesis (40). Ma et al. (41) assessed four miRNAs in CC serum samples. They examined 97 plasma samples of patients with CC. The signature of the four miRNAs, including miR-21, identified in peripheral plasma is a promising novel biomarker for diagnosing cervical cancer (41). Gao et al. (42) analyzed 140 miRNAs in 30 cervical cancer tissue samples taken in the Uyghur population, China. The qRT-PCR analysis verified that the expression of miR-21 was higher in cancer tissues than in normal tissues. The work of this group also showed that miR-21 could be a useful marker of the CC diagnosis and prognosis (42). Jia et al. (43) collected 213 serum samples from patients with confirmed CC. There were 12 markedly up-regulated serum miRNAs revealed by sequencing, and among these microRNAs, the miR-21 was identified by RT-qPCR. The results also showed that miR-21 could be used as a cervical cancer biomarker (43). Gocze et al. (33) examined 98 tissue samples with confirmed cervical lesions. This study described elevations in miR-21 expression through every disease stage from the progression of CIN1 to CC (33). Pulati et al. (44) collected 30 serum samples from HPV-16 positive patients and 30 serum samples from HPV-16 negative patients. miR-21 was down-regulated relative to HPV- 16 negative samples, and the mitogen-activated protein kinase signal pathway (mentioned above) was predicted to be a key mechanism of HPV16-related cervical cancer. This study described miR-21 as a potential marker or therapeutic target of cervical cancer (44). Zhang et al. (45) in their study defined the correlation between the level of expression miR-21 and lymph node metastasis via RASA1 expression in 89 blood samples of patients with confirmed cervical cancer. The eponymous name for RASA1 is RasGAP, which is a negative regulator of the Ras-Raf-Mek-Erk signaling pathway, as we described above. This work confirmed that miR-21 reduces RASA1 expression in cervical cancer cell lines, promotes cervical cancer cell migration, and points to miR-21 in serum as a promising biomarker of lymph node metastasis in cervical cancer (45). Sun et al. (46) showed that miR- 21 may be involved in the pathogenesis of CIN and the progression of CIN into CC (46).
Yang J. et al. (47) studies genome of 2,176 persons, including 998 healthy persons, 435 women with CIN and 743 patients with CC using TaqMan assays. They analyzed susceptibility of CIN and CC of 10 single-nucleotide polymorphisms (SNPs) located 2 kb up or downstream of miR-21. SNPs in genes of microRNAs may change level of expression or binding these molecules to their target genes (48). This work indicates that SNP rs13137 in miR-21 may affect development of CIN or CC (47). Aftab M. et al. (49) studied 460 samples of urine, serum, cervical scrape, and tissue biopsy from normal, cervical pre-cancer, and cancer patients. Expression of miR-21was upregulated in altered samples and the expression level of miR-21 in urine shows similar trends as in paired tissue biopsies, cervical scrapes and serum of precancer and cancer subjects (49).
Hoelze C.R. et al. (50) evaluated levels of miR-21 in plasma and cervical scraping, while miR-21 was up-regulated in cervical scraping samples of patients with the invasive cervical cancer. In this work miR-21 was not related to HPV infection (50). Other reviewed studies demonstrated overexpression of miR-21 also in other malignancies. Severino et al. (51) confirmed overexpression of miR-21 in metastatic and non-metastatic oral squamous cell carcinomas samples (51). Chen et al. (52) used transcription activator-like effector nucleases (TALENs) to disrupt miR-21 in cancerous cells and subsequently identified clones with no miR-21 expression. The loss of miR-21 led to subtle but global increases of mRNAs containing miR-21 target sequences. These cells become more sensitive to cisplatin and less transformed, which again points to the role of the miR-21 in carcinogenesis and also in therapeutic response (52).
Although upregulation of miR-21 has been demonstrated in the process of cervical carcinogenesis by several works, its practical use as a marker of this disease is still certain. Changes in miR-21 levels have been demonstrated throughout the whole spectrum of diseases, what can negatively affect specificity of this potential biomarker. Most circulating extracellular microRNAs are endothelial cells or blood origin (53), which leads to the question how to separate the miRNA produced only by CC cells. Questionable is also how to approach the measurement of miRNA levels, as the level of their expression measured under the same conditions may show different values (54).
miR-21, as a regulator of tumor growth, plays an important role in the process of tumor angiogenesis, tumor invasiveness, and the spread of metastasis. The studies mentioned above describe the elevation of this miR-21 in patient samples with confirmed CC. Demonstrated overexpression of miR-21 in CC cells and recognizing the signaling pathways affected by this molecule suggests using miR-21 as a suitable non-invasive biomarker of CC. Efforts to reduce global morbidity and mortality from CC, using a non-invasive test with sufficient specificity and sensitivity, force a closer understanding of the effects of miR-21 in the process of cervical carcinogenesis. However, further studies are still necessary to reach practical use of miR-21 as biomarker of carcinogenesis.
This publication was produced with the support of the Integrated Infrastructure Operational Program for the project: New possibilities for the management of serious diseases in medical and preventive care with regard to the safety of health professionals, ITMS: 313011AUA5, co-financed by the European Regional Development Fund.