









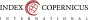

 Official publication of the Czech Society of Ultrasound in Obstetrics and Gynecology.
Official publication of the Czech Society of Ultrasound in Obstetrics and Gynecology.

Breast angiosarcomas represent the main histologic subtype of all breast sarcomas and they account for < 1% of all soft tissue tumours. Angiosarcomas arise from vascular endothelium and can develop de novo (primary angiosarcoma) or as a consequence of radiotherapy or chronic lymphedema after axillary dissection – Stewart-Trevers syndrome. Estimated incidence of postradiation angiosarcoma is 0.05 – 0.5%. Postradiation angiosarcoma typically occurs in older women (60-70 years of age) and it is a rare, aggressive tumour with a poor prognosis characterized by a high rate of local recurrence. Herein, we present a case of recurrent postradiation breast angiosarcoma with metastasis in contralateral breast.
Breast angiosarcomas represent the main histologic subtype of all breast sarcomas and they account for < 1% of all soft tissue tumours. Angiosarcomas (AS) arise from vascular endothelium and can develop de novo (primary AS) or as a consequence of radiotherapy or chronic lymphedema after axillary dissection – Stewart-Trevers syndrome (1). Estimated incidence of postradiation angiosarcoma (PRAS) is 0.05 – 0.5% (2). Primary AS is typical for younger women without previous history of breast malignancy and arises from breast parenchyma. Postradiation AS typically occurs in older women (60-70 years of age), arises from cutaneous tissue, and then infiltrates breast parenchyma (3). In 1948, Cahan et al. described criteria for postradiation angiosarcoma: angiosarcoma originates in a previously irradiated field, latent period between radiotherapy and development of the angiosarcoma must be at least 6 months, and diagnosis must be confirmed histologically (4).
The pathogenesis of postradiation angiosarcoma is related to irreversible DNA damage induced by radiation, resulting in genome instability, and by mutations of relevant cancer-related genes through direct tumour induction by radiation. Common inactivation of the p53 pathway and expression and amplification of MYC oncogene at the 8q24 region have been revealed. The MYC oncogene is well known for its role in cell proliferation, cellular differentiation, apoptosis, angiogenesis, invasion, and metastatic spread (5,6).
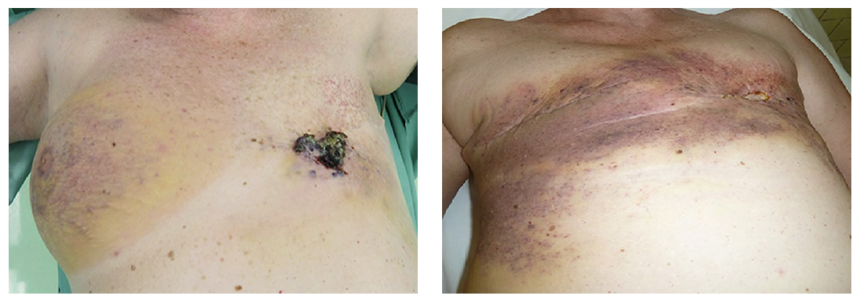
A 47-year-old woman was treated for a T1aN1Mx (stage IIA, grade 1, ER 15%, PR 40%) tubular carcinoma with the quadrantectomy of the left breast and ipsilateral axillary lymph node dissection. Adjuvant radiotherapy with total dose (TD) 70 Gy was completed 2 months after the surgery. The regular examinations were negative as long as hormonal therapy Tamoxifen was prescribed during a five-year interval after the surgery. There was no sign of recurrence, although the patient reported pain in the nipple region. Firstly, the patient was managed as having breast inflammatory disease, but due to the worsening local state, a biopsy was indicated. Pathological findings implicated a suspicion of angiosarcoma, so the mastectomy was performed. The definite diagnosis consisted of low-grade angiosarcoma with negative margins. The proliferation of atypical vascular structures was found microscopically and CD31, CD34 positivity and negativity of S-100 protein, CK7 was confirmed immunohistochemically. The endothelial lining showed mild to moderate degree of cellular atypia (cellular pleomorphia, anisonucleosis, nuclear hyperchromasia, prominent nucleoli) and increased mitotic activity 4 mf/10HPF. Five years after the left mastectomy, the patient had an injury on the right breast and the pain in the affected breast did not disappear. According to the ultrasound, mammography, and magnetic resonance findings, the recurrence appeared in the place of scar tissue after left mastectomy and a new focus was diagnosed on the right breast (Fig. 1). The right mastectomy with ipsilateral axillary lymph node dissection due to right axillary lymphadenopathy found on ultrasound altogether with the re-excision of scar tissue on the left side were performed. The histological results confirmed recurrence of the low-grade angiosarcoma and the newly diagnosed high-grade angiosarcoma on the right side with one lymph node infiltrated by metastases. The infiltratively growing proliferations of atypical complex vascular structures lined with hyperchromic endothelial cells with a variable degree of pleomorphism were found in the skin and the breast tissue. Immunohistochemical analysis of right highgrade PRAS also confirmed CD31, CD34 positivity and CK7, EMA, E-cadherin negativity. Due to the local finding, the oncologists started with farmorubicine treatment (4 cycles). Due to the spreading disease mostly on the left side and the infiltration of the ribs and thoracic muscles, (Fig. 2) the chemotherapy was changed to iphosphamid combined with uromitexan (2 cycles), subsequently to paclitaxel (17 cycles). The recurrent diseases showed resistance to paclitaxel chemotherapy, so the last line with docetaxel was introduced. The overall state of the patient was worsening with the general spread of the primary disease with infiltration of the chest wall and subsequent infection of the lesions, sepsis and intolerance to any kind of treatment. The patient died 8 years after the left-sided angiosarcoma and 2-years after the right-sided angiosarcoma was diagnosed.
Postradiation angiosarcoma of the breast is a rare complication of radiotherapy focused on the breast and chest wall. The clinical picture varies between small nodules, plaques, and teleangiectasia-like lesions of different colours and thus they can be misdiagnosed as radiotherapy dermatitis or hematoma. Therefore, the diagnosis of AS should be confirmed histologically (7). Abdou et al. (1) performed a meta-analysis of 327 SAS cases in which median time from radiotherapy (RT) to development of PRAS ranged from 51 – 180 months. Another study (8) presented a median time of 72 months.
Angiosarcomas are categorized according to the grade into low, moderate, and high-grade tumours. The higher grade along with factors such as older age and advanced stage are poor prognostic factors (9). There are only case reports, case series, and retrospective studies of PRAS available in the literature, therefore no standardized surgical and adjuvant treatment exists.
The phenomenon of high-grade transformation of metastatic right PRAS could be explained by the clonal evolution of tumor cells. The clonal evolution model assumes that genetic instability of tumor cells leads to different cell clones that contribute to tumor cell heterogeneity, subsequently acquiring additional mutations including p53 gene that promote cell proliferation generating the cells that outperform other cell populations and become the driving cell population in the tumor (10).
|
Fig. 1 Recurrence on the left side after radiotherapy |
Fig. 2 Whole breast infiltration with ulceration on the left side |
In general, according to NCCN guidelines, AS treatment consists of complete tumour excision with an adequate margin (11). The use of adjuvant RT is still controversial, because RT itself is the cause of PRAS. However, Depla et al. (8) analysed the efficacy of adjuvant RT in PRAS patients and reported a significant decrease in local recurrence (local relapse-free interval 57% vs. 34%, HR 0.46, p = 0.01) but not in overall survival. A prospective study by Smith et al. (12) reported higher rates of local control, disease free survival (DFS), and overall survival (OS) with the use of hyper-fractionated accelerated RT in the treatment of PRAS.
There is still no consensus regarding the impact on survival and the effectiveness of adjuvant chemotherapy in the treatment of PRAS because data is limited to case reports and reviews which have conflicting results (13–15). Although, adjuvant chemotherapy is mostly indicated for metastasis and local recurrence of PRAS (15). Chemotherapy regimens recommended by NCCN for AS are docetaxel, paclitaxel, and vinorelbine, another drug used is doxorubicine (11,16).
Angiosarcoma, as a mesenchymal tumour, does not typically spread via the lymphatic system, thus axillary dissection is not recommended (3). Mastectomy with negative margins remains the best surgical treatment, however, there is a small number of patients who underwent breast conserving surgery. Nevertheless, due to insufficient data, this approach cannot be recommended (7,11).
Postradiation breast angiosarcoma is a rare, aggressive tumour with a poor prognosis characterized by a high rate of local recurrence. There is a need of prospective study concerning the effectiveness of surgical and especially adjuvant treatment, but due to the rarity of this disease, it is complicated to perform such a study. Therefore, patients with PRAS should be treated by a multidisciplinary approach.