










 Official publication of the Czech Society of Ultrasound in Obstetrics and Gynecology.
Official publication of the Czech Society of Ultrasound in Obstetrics and Gynecology.

Preeclampsia (PE) is one of the most common and dangerous pregnancy complications. High mortality and morbidity of mother and foetus refer to the need of early predicition and prevention of PE. In an attempt to screen early and to prevent PE many strategies have been studied. The author of this paper worked for 2 years in the Fetal Medicine Foundation (FMF) under the supervision of Professor Nicolaides and participated in the studies SPREE, ASPRE and EVENTS which lead to establishment of a new managment of PE. The purpose of this paper is to present the development and advantages of the combined screening in the 11 – 13+6 weeks for risk calculation of PE. Up to now the main result of the first-timester combined screening was the risk calculation of aneuploidies. Nowadays the results extended to pregnancy complications as PE, growth restriction and preterm delivery. The ASPRE study presented the efficacy in the preventive treatment of the high-risk patients for PE with aspirin 150 mg taken at bed time from 12 to 36 weeks. This year FMF proposed new screening strategies which depend on the biomarker (PAPP-A vs PlGF) used in the screen calculation. The best performance of screening for PE is achieved by applying PlGF. Since PAPP-A is used routinely as part of the screening for aneuploidies, its additive value was examined in different combinations of screening strategies and confirmed its application with similar detection rate as screening by PlGF. The most recent success was a development of the first-trimester screening for PE in twins.
PE is a multisystem disorder developing during the second half of pregnancy. The ISSHP (International Society for the Study of Hypertension in Pregnancy) defines PE as hypertension (HT) arising de novo at or after 20 weeks’ gestation and proteinuria and/or maternal organ dysfunction and/or uteroplacental dysfunction. Maternal organ dysfunction includes acute kidney injury, liver involvement, neurological and hematological complications (1).
For clinical purposes PE is classified according to the time of its onset in pregnancy. Early-onset PE occurs before 34 weeks’ gestation. The condition is associated with increased maternal morbidity and the risk of intrauterine fetal death is about 4 times higher. Late-onset PE is defined as PE after 34 weeks’ gestation (2). Futher cut-off values were suggested for subdivision, such as 37 weeks. In one third of cases PE leads to delivery at < 37 weeks’ gestation (preterm PE) and in two thirds of deliveries PE occurs at ≥ 37 weeks (term PE) (3).
The incidence of PE in singletons is 2 – 5%. The rate depends on the demographic characteristics of the population (3). In twin pregnancies the rate of PE is about 9 %, which is 3-times higher than in singleton pregnancies. In fact, twins are delivered at an earlier gestational age than singletons. Francisco et al. compared the rates of PE between twin and singleton pregnancies and their results pointed to the underestimation of the relative risk of preterm PE in twins compared to singletons which was 9-times higher (4).
The traditional approach how to identify women at high-risk of PE is based on the evaluation of the medical history and risk factors from maternal demographic characteristics. With regard to PE screening two professional recommendations are being referred to: the NICE guideline (National Institute for Health and Clinical Excellence) and the ACOG (American College of Obstetricians and Gynecologists). They are based on assessing maternal risk factors (MF) and are rather similar (Tab. 1).
The NICE guideline advises pregnant women with 1 high or more than 1 moderate risk factor to take 75 – 150 mg of aspirin daily from 12 weeks until delivery. High-risk factors are any of the following: previous PE, chronic kidney disease, autoimmune disease (AID) such as systemic lupus erythematosus (SLE) or antiphospholipid syndrome (APS), type 1 or type 2 diabetes (DM) and chronic HT. Factors indicating moderate risk are: first pregnancy, age ≥ 40 years, pregnancy interval of more than 10 years, body mass index (BMI) ≥ 35 kg/m2, family history of PE and multifetal pregnancy (5).
The ACOG guideline is rather similar to the NICE recommendations and includes more risk factors in the assessment. High-risk patients for PE defined are based on the presence of 1 or more high-risk factors (previous PE, multifetal pregnancy, renal disease, AID, DM and chronic HT) or more than 1 of moderate- risk factors (first pregnancy, maternal age ≥ 35, BMI > 30, family history of PE, sociodemographic characteristics, and personal history factors). The ACOG recommends low-dose aspirin (81 mg/day) in women at high-risk of PE. The prophylaxis should be initiated between 12 weeks and 28 weeks of gestation (optimally before 16 weeks) and continued daily until delivery (6).
In 2017 O’Gorman et al. compared the performance of screening for PE based on the above mentioned recommendations. The NICE guideline detected 39% (95% CI, 27–53%) of preterm PE at 10% false positive rate (FPR). Screening according to the ACOG recommendations detected 90% (95% CI, 79–96%) of PE < 37 weeks at 64% FPR. (7) The results reflected the disadvantage of both guidelines: the NICE results showed low DR and the ACOG screening had a high FPR.
Within the last decade under the supervision of Professor Nicolaides FMF has been devoted to create screening programme for PE. The author of this paper has participated in the studies SPREE (Screening ProgRamme for prE-Eclampsia) and ASPRE (ASpirin for evidence-based PREeclampsia prevention) which lead to establishment of a new managment of PE in singletons. The approach to risk assessment
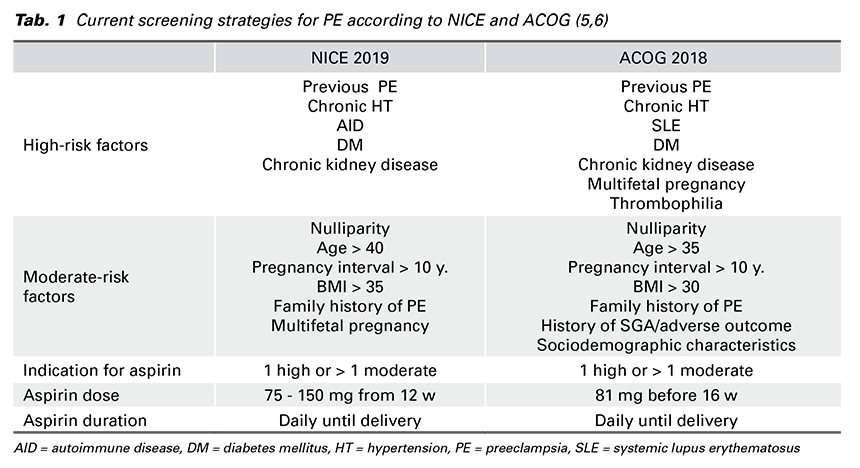
was to apply Bayes’ theorem to combine the a-priori risk from MF with the results of various combinations of biophysical and biochemical measurements. This method assumes that if the pregnancy was to continue indefinitely all women would develop PE. The effects of variables (from MF + biomarkers) is to modify the mean of the Gaussian distribution of gestational age at delivery with PE. Consequently, pregnant woman has an estimated patient-specific risk of developing each form of PE (early or late-onset, preterm or term PE). Whether the patient developes PE or not before a specified gestational age depends on competition between delivery before or after development of PE. In low-risk pregnancies the gestational age distribution is shifted to the right (Fig. 1) with the implication that in most pregnancies delivery occurs before development of PE. In high-risk pregnancies the distribution is shifted to the left. The smaller the mean gestational age is, the higher is the risk for PE (8).
The MF which increase the risk of PE include: advancing maternal age, increasing weight, Afro-Caribbean and South Asian ethnicity, HT, DM and SLE or APS, in vitro fertilization (IVF) and family history or personal history of PE (9).
In the screening programme it is essential to standardize the measured values of biomarkers which can be achieved by using multiples of the normal median (MoM) values. The biophysical markers include mean arterial pressure (MAP) and uterine artery pulsatility index (UTPI). There were many analysed biochemical markers and few worth to mention are pregnancy associated plasma protein-A (PAPP-A), placental growth factor (PlGF), serum soluble fmslike tyrosine kinase-1 (sFLT-1) or cell free DNA. Futher studies showed that inclusion of cell free DNA and sFLT-1 does not improve the performance of screening (10,11,12,13).
The advantage of assessing a patient-specific risk of PE is that it has an individualized approach. In comparison with other screening methods, the FMF screening includes both MF and biomarkers and takes in account that PE can develop at any gestational age.
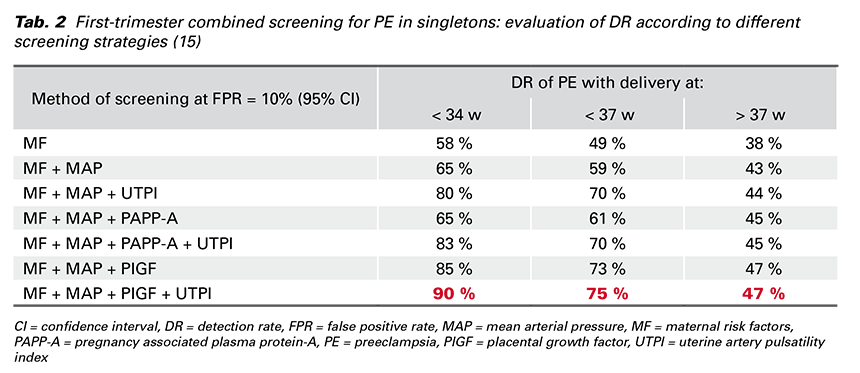
In 2017 Professor Nicolaides introduced a new protocol for the screening programme for PE at 11-13 weeks’ gestation in a prospective multicenter cohort study called SPREE. MF and biomarkers (MAP, UTPI, PAPP-A and PlGF) were recorded according to standardized protocols and the results validated that the most effective screening for PE is calculated by a combination of MF + MAP + UTPI + PlGF (Tab. 2). This screening programme predicts about 90% of early-onset PE, 75% of preterm PE and 45% of term PE at FPR of 10% (14,15).
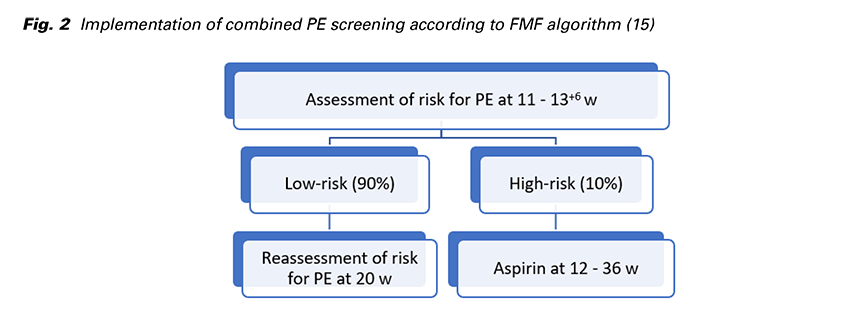
FMF proposed a strategy to screen the whole population for PE and subsequently to devide patients into two management pathways (Fig. 2): High-risk group (risk for preterm PE of ≥ 1 in 100) constitutes about 10% of the total and contains 75% of those that develop preterm PE. Treatment with aspirin (150 mg/day) taken at bed time from 12 to 36 weeks reduces the risk of preterm PE by 62%. Low-risk group (risk for preterm PE of < 1 in 100) constitutes 90% of the total and can be reassured that development of PE is unlikely, but the risk reassessment is required at 22 weeks’ gestation (16).
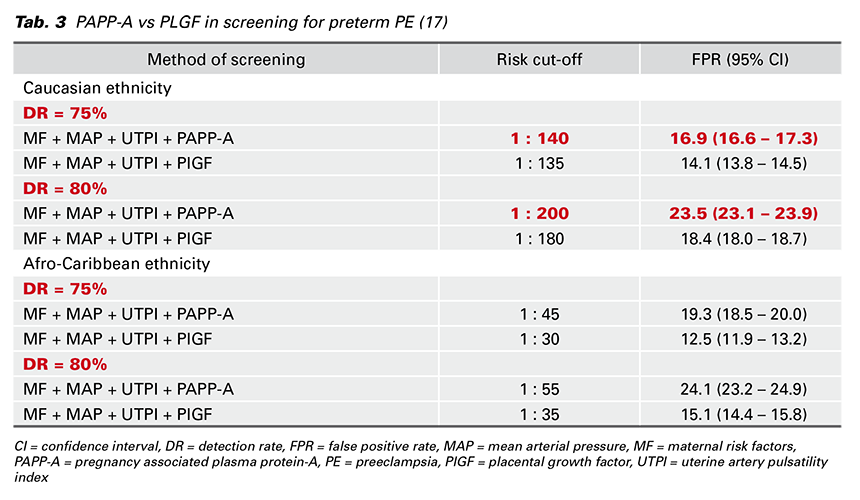
The above mentioned strategy identifies the highrisk group by a combination of MF, MAP, UTPI and PlGF. However, an alternative biochemical marker is PAPP-A, which is used widely as part of early screening for trisomies. Mazer Zumaeta et al. examined the additive value if PAPP-A rather than PlGF was used for the first-trimester screening. Obviously, their results confirmed that screening by PlGF (MF + MAP + UTPI + PlGF) is superior to that one by PAPP-A (MF + MAP + UTPI + PAPP-A). For instance, the DR in screening by PlGF was 7.1 % (95% CI, 3.8–10.6%) higher than in screening by PAPP-A at the same FPR (10%). However, to achieve a desired DR of PE Mazer Zumaeta et al. defined the risk cut-off and FPR which vary according to the ethnicity and whether the PlGF or PAPP-A is measured. In the Tab. 3, I propose different screening strategies. In the following example I use Caucasian ethnicity which is the most common in Slovakia: in the screening by PlGF, if the desired DR of preterm PE is 75%, the risk cut-off should be 1 in 136 and the FPR would be 14.1 %. In the screening by PAPP-A with the same DR the risk cutoff should be 1 in 140 and the FPR would be 16.9% (17). To summarize, the study offers the inclusion of PAPP-A into the first-trimester screening for preterm PE. The FPR is slightly higher and depends on the desired DR and ethnicity.
The rate of PE is not reduced by bed rest, restriction of physical activity or dietary manipulations, including restriction of salt intake or supplementation with magnesium, zinc, folate, vitamins C, D and E or fish oil (18).
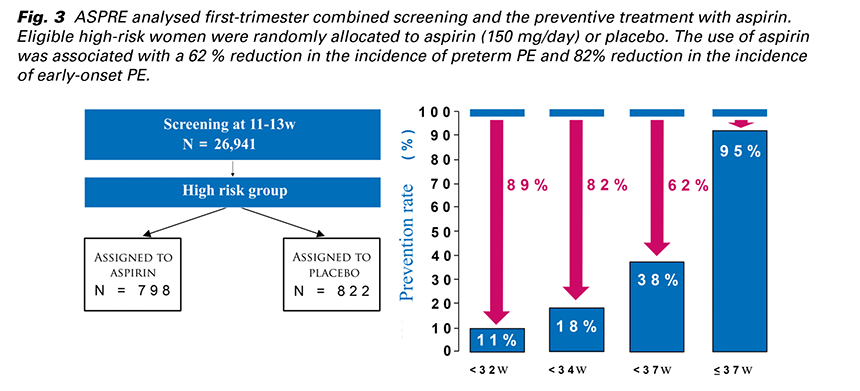
The most successful study about the prevention of PE by aspirin was ASPRE from 2017. It was an international multicenter prospective double blinded study which analysed first-trimester combined screening and the preventive treatment with aspirin. The screening was carried out at 11 - 13 weeks’ gestation by the FMF algorithm in about 27,000 singleton pregnancies. (Fig. 3) Eligible high-risk women were randomly allocated to aspirin (150 mg/day) or placebo. The use of aspirin was associated with a 62% reduction in the incidence of preterm PE and 82 % reduction in the incidence of early-onset PE (19).
Secondary analysis of the ASPRE trial showed that the beneficial effect of aspirin depends on compliance. The reduction in incidence of preterm PE may be about 75% in those with compliance of ≥ 90% and only 40% in those with compliance of < 90%. Therefore, it is inevitable to motivate a patient to adhere to the aspirin-treatment. Noncompliance is a great problem which can lead to the development of PE. In addition, secondary analysis showed that aspirin may not be useful in the prevention of preterm PE in patients with chronic HT (20). Despite lacking proof many experts recommend prescribing aspirin to high-risk patients with chronic HT.
Recently, FMF came with the first-trimester screening programme for PE in twins. Two datasets of prospective non-intervention multicenter screening studies were used. The first dataset was from the EVENTS trial (Early vaginal progesterone for the preVention of spontaneous prEterm birth iN TwinS) which was conducted from 2017 to 2019 and included 1,798 patients. The second dataset was from prospective screening for adverse obstetric outcomes in women attending their first routine hospital visit at King’s
College Hospital and Medway Maritime Hospital, UK, from 2006 to 2015 and included 3,938 patients (21).
The approach to risk assessment for PE screening in twins was similar to that one in singletons: the same a-priori risk based on MF and medical history was used. The measured values of biomarkers were converted to MoMs. The posterior distribution of gestational age at delivery with PE was obtained using Bayes’ theorem by multiplying the a-priori risk by the likelihood function from biomarker MoM values (22). Consequently, FMF developed a model in which the effect of twins shifted the distribution of gestational age of delivery with PE to the left. The shift to the left was greater if the prior mean was high and less if the mean was low (21).
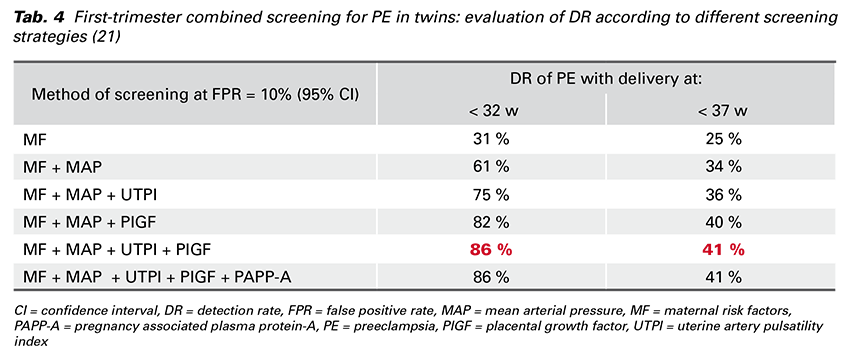
The best performance of the first-trimester screening for PE in twins is achieved by a combination of MF, MAP, UTPI and PlGF (Tab. 4) and there is no additional contribution from PAPP-A. The DR is better by calculating the risk for PE < 32 weeks’ gestation than the risk for PE ≥ 37 weeks’ gestation (21).
In singletons the first-trimester screening, which use the combination of MF, MAP, UTPI and PlGF and cutoff value 1:70, identifies 90% women with PE at < 32 weeks’ gestation and 75% women with preterm PE at 10% FPR. Screening in twin pregnancies with the same risk cut-off would detect all cases of preterm PE but at FPR of 94%. This model would not meet the criteria of effective screening and the FPR should be minimized. This could be achieved in twins if the cut-off risk is set to 1 in 15, then the DR of preterm PE would be 75% and the FPR of 40%. In addition, there is so far no clear evidence of beneficial aspirin-treatment of these high-risk patients. Therefore Professor Nicolaides suggests a conduction of a major randomized trial to investigate the possible effect of aspirin in the prevention of preterm PE in twins (21).
Nowadays there are many studies which refer to the early screening and prevention of PE. Some of them are based just on assessing patients risk factors and their disadvantage is either low DR or high FPR. On the other hand, the combined first-trimester screening for PE, which was established by FMF, has an individualized approach and assess a patient-specific risk for PE according to the MF with the results of various combinations of biophysical and biochemical measurements.
FMF developed an algorithm for risk calculation for PE and the most effective screening is calculated by a combination of MF + MAP + UTPI + PlGF. However, PAPP-A is used widely as part of early screening for trisomies and last year FMF examined its additive value if it is included into the first-trimester screening rather than PlGF. They confirmed its additive value and defined the risk cut-off and FPR which vary according to the ethnicity.
High-risk patients are recommended to the treatment with aspirin (150 mg/day) taken at bed time from 12 to 36 weeks. Such a treatment reduces the risk of preterm PE by 62% what consequently leads to the reduction of neonatal and maternal morbidity.
Recently, FMF came with the first-trimester screening programme for PE in twin pregnancies. There is so far no clear evidence of beneficial aspirin-treatment of this high-risk patients. To investigate the possible effect of aspirin in the prevention of preterm PE in twins, there is a need of new trials which should be idealy prospective, randomized and double blinded.