









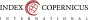

 Official publication of the Czech Society of Ultrasound in Obstetrics and Gynecology.
Official publication of the Czech Society of Ultrasound in Obstetrics and Gynecology.

Objective: Case description of negative histopathology result after salpingectomy due to tubal ectopic pregnancy.
Methods: Own observation and review of the literature.
Conclusion: A negative histopathology result could occur even in the case of correctly diagnosed and surgically treated tubal ectopic pregnancy.
Diagnosis of a tubal ectopic pregnancy (TEP) may be tricky even for skilled sonographers, mostly due to the very early first ultrasound scans. The final decision in managing such cases is taken by considering the clinical state of the patient, the serum levels of hCG and the ultrasound findings. The risk of intervention has to be balanced against the risk of a delay in the treatment, which might result in a more aggressive treatment than would have been necessary if the intervention had occurred sooner (1). In the case of salpingectomy, the histopathology analysis is performed to confirm the diagnosis, as a standard procedure after each surgery. However, the histopathology result might not be consistent with the prior sonographic and perioperative findings. This report presents a case of TEP in which histopathology was negative after unilateral salpingectomy
A 31-year-old woman, nulligravida, with a history of prior unilateral salpingectomy due to pelvic inflammatory disease, undergoing assisted reproductive therapy (ART) for primary infertility (immunologic factor – positive anti-zona pellucida antibodies), was submitted to controlled ovarian hyperstimulation, oocyte pick-up, intracytoplasmic sperm injection and transfer of one zona-free fresh embryo. She was examined in our clinic on day 13 after embryotransfer (ET) due to a prior short episode of vaginal bleeding, with no bleeding during the day of the check-up. Vaginal examination showed no bleeding or other abnormalities and the transvaginal ultrasound (TVUS) showed an empty uterine cavity with a homogenous 8 mm endometrium, no adnexal masses, and no free pelvic fluid collection. The patient was asymptomatic, with serum hCG level of 142 U/l on day 12 after ET. Due to pregnancy of unknown location (PUL), the patient was scheduled for a repeated serum hCG level check-up on day 16 after ET and for a TVUS on day 17 after ET. Serum hCG level on day 16 after ET was 997 U/l. Patient’s clinical evaluation on day 17 after ET showed no pain, no vaginal bleeding, and no signs of peritoneal irritation. However, TVUS revealed an empty uterine cavity with a homogenous 9 mm endometrium, without visible intrauterine gestational sac and a 16 mm right adnexal mass (blob sign), suggestive of right tubal ectopic pregnancy (Fig. 1).
On the same day, after TVUS confirmation by two other sonographers, the patient was submitted to explorative laparoscopy, revealing normal ovaries, missing left Fallopian tube after prior salpingectomy, approx. 30 ml of blood in Douglas pouch and a right Fallopian tube mass, which supported the diagnosis of TEP. Subsequently a unilateral salpingectomy was performed and the blood from Douglas pouch was removed by suction. Dilation and curettage was not performed. Serum hCG level dropped the next day to 185 U/l and hCG was negative one month after the surgery.
However, the histopathology result showed no correlation with the diagnosis of TEP, despite the thorough analysis of the whole 75 mm Fallopian tube. The analysis revealed just a blood clot in the lumen of the tube, failing to detect any signs of chorionic villi in the entire sample.
After a proper consultation of the patient, one (of two remaining) cryopreserved zona-free embryo was transferred two months after the surgery, resulting in an ongoing intrauterine pregnancy.
During the monitoring of a PUL, the diagnosis of TEP is made more often after the second look ultrasound scan, and not on the first contact ultrasound scan. The reason for this is not due to oversight on the first contact ultrasound scan but because the first scan is performed during the very beginning of the pregnancy development (2). The ultrasound criteria for diagnosing an ectopic pregnancy did not change, the accuracy of TVUS in detecting TEP is high and reaches sensitivity of 87-99% and specificity 94-99,9% (3). Pregnancies resulting from ART, as well as history of prior pelvic inflammatory disease (both criteria present in our patient) are associated with an increased risk of developing a TEP (4).
Excluding the frustrating result of the histopathology, one can consider this case as an exemplar case of managing a tubal ectopic pregnancy. However, the result of the histopathology could be especially frustrating for the clinician and should be carefully and correctly consulted with the patient in order to exclude future legal issues. After reviewing the literature, we can state that several previous studies of the diagnostic accuracy of TEP did not report any cases of a positive surgical diagnosis of TEP that was not confirmed histologically (5,6,7). Just one single retrospective cohort study, concerning correlation of the outcomes of histological examinations with surgical findings and pre-operative ultrasound scans, has shown that failure to confirm a TEP is a fairly common outcome at histological examination, occurring in under 5% of cases (8). The reasons of histological examination failure to confirm TEP could be: 1) the incorrect sonographic and surgical diagnosis (true false positive on both ultrasound and surgery), or 2) TEP may have been present but omitted from the surgical specimen or missed during the analysis in the histopathology lab – either because the product of conception had already miscarried from the tube and been removed by suction with blood in the pelvis or because the tissue containing chorionic villi was not included in the sample sent to the lab (due to nurse or surgeon error – ectopic pregnancy may be smaller than the associated hematosalpinx).
Farahani et al. (8) analyzed 44 cases of TEP with no histological signs of chorionic villi in the specimen. 16 cases revealed pathologies of the Fallopian tube other than TEP, however 3 of these cases needed second surgery due to ongoing ectopic pregnancies. 28 cases showed hemorrhage in the Fallopian tube as an indirect evidence of TEP. In 5 of these cases with hemosalpinx, the intraluminal trophoblast alone was also present. In 2 of 23 cases with hemosalpinx alone, the ectopic pregnancy persisted and second surgery was necessary. Farahani et al. also analyzed risk factors for negative histology. Women with negative histology had a lower gestational age, smaller ectopic pregnancies and lower hCG levels compared to women with positive histology.
These results suggest that indirect histological signs of TEP can be helpful. In the case of hemosalpinx without the presence of chorionic villi or the intraluminal trophoblast, the risk of necessary second surgery is approximately 9%, whereas the risk of necessary second surgery in the case without indirect evidence of TEP is approximately 19%. The small number of cases in the study (8) impels one to take this result with caution.
There are biological reasons supporting the assumption that the negative histology of TEP specimen can be more likely due to the biological complexity of life than the result of medical error. First, all cases of negative histology described in study (8) as well as our case were associated either with indirect evidence of TEP or with pathologies affecting microscopic appearance of the Fallopian tube. Second, early resorption of the embryo is an evolutionary conserved strategy preventing later development of unviable embryos. This process is triggered by endogenous apoptosis of the embryo, and by the mother’s inflammatory cells (9). Third, the quality of chorionic villi belonging to the ectopic pregnancy is overall bad, including delayed development, changes in trophoblast, and poorer branching (10). Taken together, one can hypothesize that ectopic pregnancy can be, at least in some cases, prone to mechanical disintegration or to an easier release from the wall of the Fallopian tube during pathological development of TEP (or during surgical manipulation). Therefore, certain histological signs of pregnancy (i.e. embryonic structures or chorionic villi), can be lost in the blood clot or during surgical manipulation. Despite lack of studies, careful analysis of free blood clots is recommended in the textbooks of surgical pathology, because only few embryonic cells can occur in the sample (11,12).
Our case taken together with the result of study (8) suggest that the risk of necessary second surgery after negative histology of first specimen is approximately 11%. For better understanding of this frustrating phenomenon, further research based mainly on retrospective analysis of similar cases is strongly needed.
In light of growing ART and consequent increase in TEP risk, all physicians should be aware of the possibility of negative histology report after salpingectomy. In case TEP is suspected, an adequate counselling of the patient is needed prior to each explorative laparoscopy. The informed consent of the woman undergoing explorative laparoscopy due to possible TEP should include counselling about the risks of negative laparoscopy as well as the inability to identify the cause of persistence of trophoblastic tissue and/or the failure of histopathological confirmation after salpingectomy.