










 Official publication of the Czech Society of Ultrasound in Obstetrics and Gynecology.
Official publication of the Czech Society of Ultrasound in Obstetrics and Gynecology.

We report a unique case of a rare prenatal diagnosis of transient myeloproliferative disorder mimicking congenital leukemia, since child had no features associated with Down syndrome. Leukemic process is usually detected at birth or very shortly thereafter. Diagnosis of congenital leukemia was suspected in third trimester of pregnancy. During ultrasound examination we found hydrops foetus, pericardial effusion, hypoechoic hepatosplenomegaly with “starry night” appearence and polyhydramnios. After delivery by Caesarean section in 36th week of pregnancy, peripheral blood examination revealed leukocytosis and thrombocytopenia. Bone marrow aspiration showed 20% of myeloid blasts typical for megakaryoblastic leukemia/transient myeloproliferative disorders. Trisomy 21 was present only in blasts and GATA1 mutation was also confirmed. Based on an early prenatal suspicion and quick and intensive postnatal intervention patient survived.
Congenital leukemia is extremely rare disease, which develops in utero. Congenital leukemia are those detected at birth or up to 30 days after delivery. Incidence is reported to be 1 – 5 cases in million live births (1). Many cases are unrecognized, because of inadequate histological examination of the stillborn infant and the placenta. Most cases of congenital leukemia are associated with transient myeloproliferative disorders in Down syndrome with mutation in gene GATA1 or other chromosomal anomalies (2). Congenital leukemia in phenotypically and cytogenetically normal children whose blasts had acquired trisomy 21 and GATA1 mutation has been reported in the literature rarely. Congenital leukemia is life-threatening disease with poor prognosis.
This case confirms the difficulties in differentiation between congenital leukemia and transient myeloproliferative disorder presented in literature. Therefore this diagnosis is important to have in mind also in prenatal care.
In our case we report 28 year old mother who was primigravida, nulipara with A blood type, Rh positive. In her family history there was no serious hereditary disorder, no malignity. She did not take any regular medication. In her gynaecologic history she was observed for primary sterility, why she underwent laparoscopic surgery including ovarian drilling and chromopertubation. There was no exposure to possible mutagens or teratogens.
First trimester screening showed vital monofetal pregnancy, measurement of CRL 62 mm response to 12/+4 week of gestation. Nuchal translucency was 0.8 mm, nasal bone was detected and there was normal quantity of amnion fluid – normohydramnios. All scans were performed with the Voluson E6 BT13 (GE Healthcare, Zipf, Austria) equipped with a convex 4–8 MHz abdominal transducer (RAB 4–8L). Free beta human chorionic gonadotrophin (1.421 MoM) and plasma associated plasma protein A (1.221 MoM) were obtained in the 12th week. Down’s syndrome risk was very low (1:10 248). Biochemical and serological screening was negative (TORCH negative). Second trimester morphology ultrasound screening (20th week scan) was without any special pathologic signs.
Fetal ultrasound screening performed in 30th week of gestation was normal. There was normal volume of amniotic fluid. Estimated fetal weight (EFW) was 1662 grams, Doppler flowmetry of the fetal umbilical artery showed normal blood flow class (BFC). In 34th week of gestation there was normohydramnios, EFW 2750 grams, Doppler of the fetal umbilical artery showed normal BFC as well. At 36 weeks (35+1) patient came to hospital due to sporadic uterine contractions. Transvaginal ultrasound measurement of cervical length was normal. We observed mild polyhydramnios with amniotic fluid index 26. In fetus we discovered mild hepatomegaly. Complete blood count was normal, serological screening for TORCH infections was negative and red blood cell antibody screening was negative. We repeated the ultrasound examination after 3 days (35/+4 weeks). We noticed significant increase of amniotic fluid volume – amniotic fluid index 35, EFW was 3073 grams. Fetal umbilical and middle cerebral arterial Doppler assessment was reported to be normal. We found hepatosplenomegaly with typical sonographic appearance of the liver parenchyma with bright echogenic dots throughout of hypoechoic liver parenchyma (starry sky appearance) (Fig. 1). Other ultrasound findings were edema of the skin and pericardial effusion what referrers to mild generalised fetal hydrops. Serological tests for TORCH and red blood cell antibody screening were negative again.
Due to fast progression of the hepatosplenomegaly and hydrops fetalis, a Cesarean section at 35+6 weeks of pregnancy was performed and a female neonate of 3150 g and 48 cm with Apgar score 5/8/6 was delivered. Neonate had respiratory distress and enlarged abdomen (Fig. 2). Newborn´s health condition required endotracheal intubation and mechanical ventilation. Postnatal blood examination revealed leucocytosis with a total leucocyte count of 160 x 109/l and thrombocytopenia with a platelet count of 20 x109/l. Peripheral blood and bone marrow smears showed typical myeloblast for children with Down syndrome (Fig. 3). Imunophenotyping of bone marrow revealed blasts with expressions as follows: CD45dim+ CD117+ CD34 partial heterogenous + HLA-DRneg, CD13+, CD33+, CD7+, CD4+, CD42b+, CD41/CD61+, CD71+, typical for transient myeloproliferative disorder. Cytogenetics with FISH revealed trisomy 21 (Fig. 4). Further molecular investigations confirmed mutation of exon 2 of GATA1 gene. After confirmation of transient myeloproliferative disorder, the newborn underwent three cycles of low-dose cytarabin chemotherapy. During the treatment child has developed severe life threatening infectious complications and very severe hepatopathy. Aggressive supportive treatment in intensive newborn unit was needed. Currently, child is 3 years old and is regularly seen by hematologist with monitoring of CBC to detect any changes for possible leukemia. Child has no signs of Down syndrome and has normal mental and physical development.
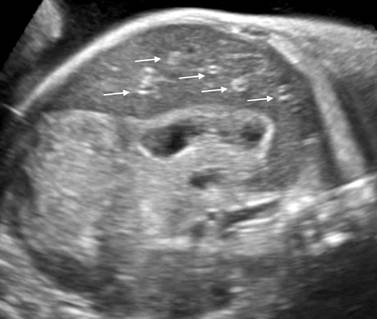
Fig. 1 Transabdominal oblique view of the fetal abdomen with typical sonographic appearance of the liver parenchyma (left lobe) with bright echogenic dots (arrows) throughout of hypoechoic liver parenchyma (starry sky appearance)
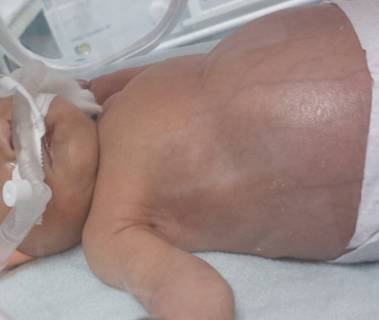
Fig. 2 Postnatal picture of the newborn with markedly enlarged abdomen (disproportion between the chest and abdomen)
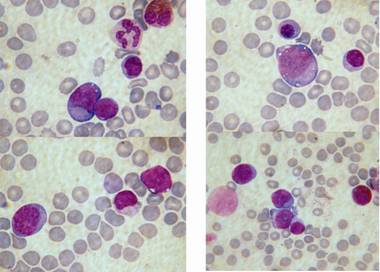
Fig. 3 Blasts in peripheral blood and bone marrow smears
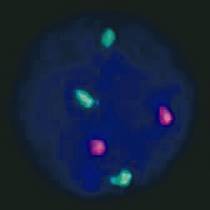
Fig. 4 Trisomy 21 in blast revealed by cytogenetics with FISH (Fluorescence in situ hybridization)
We described a case of a patient with congenital leukemia features detected in utero. Based on prenatal ultrasound examinations and early postpartum treatment, this three-year old child survived and has normal psychosomatic development. This is the unique case of transient myeloproliferative disorder which is not associated with Down syndrome. Trisomy 21 was present only in blasts.
Leukemias of myeloblastic origin in newborns are more frequent than lymphoblastic leukemias in contrast to pediatric leukemias (3). Most cases of CL are associated with Down syndrome. Other anomalies associated with congenital leukemia are Noonan syndrome, Mosaic trisomy 21, translocation of gene MLL (Mixed lineage leukemia), which is responsible for regulating hematopoietic precursors count (4,20,22). In our case, mutation of exon 2 of GATA1 gene (Xp11, 13) was found. GATA1 is essential for the differentiation regulation, proliferation and apoptosis, during erythropoiesis and megakaryopoiesis (2,5). Somatic mutations in gene GATA1 cause transient myeloproliferatic disease (TMD). Transient myeloproliferative disease has been identified by its unique characteristics of paucity of leukemic blasts in the marrow, variable pancytopenia, a propensity for mild to life-threatening hepatic infiltration. Although transient myeloproliferatic disease can be fatal in 10% of patients, it most often resolves spontaneously within 4 – 10 weeks without any intervention help. Treatment is reserved only for life threatening cases (hyperleucocytosis, sepsis, severe bleeding, liver fibrosis, etc.). By the 5th year of age, 20% of patients progress to acute megakaryoblastic leukemia (1,6). 5 – 10% of children with Down syndrome have transient myeloproliferatic disease, but rarely, it can be diagnosed also in newborns without Down syndrome (2,7,16,22). Congenital leukemia in phenotypically and cytogenetically normal children whose blasts had acquired trisomy 21 and GATA1 mutation is uncommon (3,8,9,19). There was reported a case of transient myeloproliferative disorder without any detectable cytogenetic anomalies involving chromosome 21 or GATA1 mutations (16).
In our case, the blasts were positive for CD 41/61 and 42b, as well as for CD 117. Stem cell factor CD 117 is present on approximately 4% of normal bone marrow mononuclear cells and in acute myelogenous leukemia and chronic myelogenous leukemia in myeloid blast crisis, but rarely, in acute lymphoblastic leukemia (7).
Etiological considerations in congenital leukemia have included genetics, chromosomal defects, intrauterine environmental insults, socioeconomic status, viral infections as EBV, maternal consumption of DNA topoisomerase-II-inhibitor-containing foods and exposure to radiation during pregnancy (1).
Diagnosis of congenital leukemia includes proliferation of immature white cells, infiltration of these cells into extra hematopoietic tissues, absence of any other disease that can cause leukemoid reaction mimicking leukemia (4).
In differential diagnosis it is important to distinguish congenital leukemia from other leukemoid reactions that cause hepatomegaly and elevated level of white blood cells. Obstetrical causes of leukemoid reaction can be a response to hypoxia or serologic conflict. In differential diagnosis we have to exclude congenital infections such as CMV, EBV, Syphilis, Toxoplasmosis, herpetic viruses, congenital HIV and bacterial infections such as Listeria monocytogenes, Tuberculosis, Brucellosis. Haematological causes are haemolytic disease of the newborn (Rh and ABO incompatibility), Aplastic and Fanconi anemia, or megaloblastic anemia. We also have to differentiate CL from malign disease as neuroblastoma or Langerhans cell histiocytosis (10). Congenital leukemia is typically presented with hepatosplenomegaly, leukemia cutis – petechiae, ecchymosis and/or hyperleucocytosis (Fig. 1). Leukemic infiltrates can affect lungs and other organs as well (12).
Prognosis of congenital leukemia is poor and it is dependent on the stage of the disease in time of birth. On the other hand the prognosis of transient myeloprolferative disorder is favourable. The current case shows the importance of early prenatal determination and necessity of early intervention. Prenatal diagnosis is very rare. In prenatal diagnostic we can find hydrops fetalis (80%), pleural effusion, ascites, skin edema and hypoechoic hepatosplenomegaly (90%) in ultrasonography examination associated with abnormal extramedullary hematopoiesis in fetal liver (13,14,15,17). In our case we have found these signs, and in addition, also pericardial effusion and polyhydramnios. Some authors referred other prenatal findings in congenital leukemia such as elevation of pulsatility index of the umbilical artery (normal in our case), enlarged fetal trunk, hydropic placenta and splenomegaly in ultrasound, pathologic cardiotocograph - sinusoidal fetal heart rate pattern, late decelerations (12,18,19,20). In prenatal diagnosis of transient myeloproliferative disorder an elevated delta OD 450 levels (indicator of the fetal liver function) has been found by amniocentesis (21). To confirm the diagnosis it is recommended to perform cordocentesis and PCR examination. In our case in karyotype of the blasts were trisomy 21. Cells other than blasts has showed as normal. Therefore, transient myeloproliferative disorder cannot be completely ruled out even though karyotype by amniocentesis is normal (19).
Mostly congenital leukemia/transient myeloproliferative disorder is diagnosed at birth or within few days after. We need to keep in mind this severe diagnosis during prenatal period as well. Up to 11% of non-immune hydrops fetalis have hematologic or lymphatic etiology (23). It is important to diagnose this disease as soon as possible in viable pregnancy to prevent stillbirth as well as to reduce neonatal morbidity and mortality. Early prenatal detection is important as it permits parental counselling, and when appropriate, prenatal therapeutic support. It is possible that supportive prenatal therapy may facilitate prolongation of gestation and enable postnatal therapy to be initiated. There is currently insufficient evidence to make recommendations regarding prenatal supportive therapy in transient myeloproliferative disorder (24).
This work was supported by a research grant from the Czech Ministry of Health (Grant No. RVO-VFN64165).